Lipids, Oxidative Stress, Inflammation, and Cardiometabolic Disorders: An Integrated Approach
| Received 06 Aug, 2025 |
Accepted 10 Oct, 2025 |
Published 20 Oct, 2025 |
Critical, interconnected biological processes involved in cardiometabolic diseases’ pathophysiology, like atherosclerosis, type 2 diabetes, and metabolic syndrome, include lipids, oxidative stress, and inflammation. Alteration of lipid metabolism, i.e., high Low-Density Lipoprotein (LDL) and low High-Density Lipoprotein (HDL), results in lipid oxidation and deposition within vascular tissues. By generating Reactive Oxygen Species (ROS), which damage endothelial function and induce inflammatory signaling, oxidized lipids increase oxidative stress. Chronic inflammation, created through pro-inflammatory cytokines and activated immune cells, creates a vicious cycle of metabolic disease when it further interferes with insulin signaling and lipid homeostasis. Clinically, this investigation supports the use of early oxidative stress assessment, inflammatory screening, and lipid profile to inform risk assessment, preventative care, and treatment strategies. To decipher the multifaceted aetiology of cardiometabolic disease, an integrative strategy is required that accounts for the interplay between oxidative stress, inflammation, and lipid metabolism. Multi-targeting drugs, including lipid-lowering agents, antioxidant supplements, and anti-inflammatory treatments, can retard the progression of the disease, according to new research. Future directions for identifying new biomarkers and treatment targets are provided by omics and systems biology approaches. To develop targeted and efficient prevention and treatment protocols for cardiometabolic diseases, a deep knowledge of these interacting pathways is essential, thus underlining the importance of multidisciplinary investigation and integrative clinical approaches.
| Copyright © 2025 Samson et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
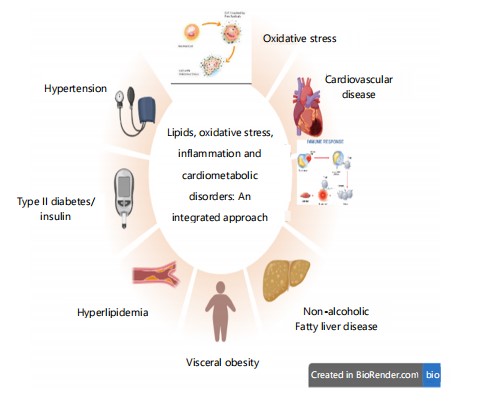
Cardiometabolic disorders (CMDs) constitute a cluster of diseases with interrelated conditions including hypertension, Insulin Resistance (IR), dyslipidemia, central obesity, and type 2 diabetes mellitus (T2DM), which, cumulatively, elevate the risk of cardiovascular disease (CVD) and metabolic ill1 in Fig. 1. The conditions share underlying disease mechanisms, including chronic inflammation, endothelial dysfunction,
|
By highlighting their interrelated molecular pathways, the integrated approach draws attention to important linked illnesses such as cardiovascular disease, non-alcoholic fatty liver disease, hypertension, type 2 diabetes, hyperlipidaemia, and visceral obesity and hormonal imbalance, typically triggered by modifiable lifestyle determinants like unhealthy diet, physical inactivity, and tobacco smoking. The principal cardiometabolic risk factors, include hypertension, dyslipidemia, obesity, IR, and glucose intolerance, are major contributors to the pathogenesis and course of both metabolic disease and CVD1.
Metabolic syndrome (MetS) and CMD are two highly similar terms, with a set of overlapping features, but with differences in scope and use in the field. Both define a cluster of risk factors, including hypertension, insulin resistance, dyslipidemia, and central obesity, that contribute to cardiovascular disease and type 2 diabetes. However, MetS is a diagnostic construct that is defined by the presence of three or more of five standardized criteria (e.g., elevated waist circumference, raised triglycerides, low HDL cholesterol, raised blood pressure, and fasting hyperglycemia), which is largely used to describe individuals at heightened metabolic risk1. On the other hand, CMDs is a more all-encompassing, broader term encompassing not only the components of MetS but also other related diseases like atherosclerosis, non-alcoholic fatty liver disease, and chronic inflammation2,3. Thus, while MetS is a subset with well-defined clinical cut-offs, CMDs are a larger category of interconnected conditions that affect cardiovascular and metabolic health.
The CMDs have long been associated with traditional risk factors that consist of ageing, genetic predisposition, obesity, hypertension, diabetes mellitus, dyslipidaemia, and smoking4. The traditional factors continue to play a significant part in the aetiology and development of CMDs, as do non-cardiac comorbidities such as liver disease and chronic kidney disease (CKD). The CMD burden has increased globally as a result of the expanding obesity and type 2 diabetes mellitus (T2DM) epidemics, mainly due to physical inactivity and unhealthy diets5. High consumption of sodium, trans fatty acids, and processed meat, along with energy-dense diets with low intake of fruits, vegetables, and whole grains, are the main determinants of the rising burden of these metabolic disorders6.
An increasing amount of evidence underscores the contribution of new environmental and lifestyle determinants in addition to traditional risk factors. An increasing number of studies have implicated the pathophysiology of CMDs in exposure to ambient environmental pollutants, and in particular fine particulate matter (PM2.5). By releasing pro-inflammatory cytokines, PM2.5 can stimulate immune cells, cause systemic inflammation, and further endothelial dysfunction7. Oxidative stress (OS), increased generation of reactive oxygen species (ROS), and disturbances in lipid metabolism, particularly high levels of free fatty acids and oxidized lipids such as 7-ketocholesterol very frequently associated with this inflammatory cascade8. These lipids were shown to accumulate in macrophages and aortic plaques in animals that were exposed to particulate matter with a diameter of 2.5 (PM2.5), lending credence to the fact that similar processes may be involved in humans who are exposed to air pollution9.
Concurrently, micro and nanoplastics (MNPs) are novel environmental contaminants with profound cardiometabolic consequences; recent research identified MNPs in arterial plaques, which were linked to higher cardiovascular events like myocardial infarction and stroke, implicating their role in augmenting vascular damage and systemic inflammation10-12. Climate change and global warming are also increasingly being acknowledged as indirect but powerful determinants of CMDs: Rising global temperatures increase exposure to heat extremes, worsen food insecurity, and increase psychological stress through migration and displacement, all of which can negatively impact cardiometabolic health5, and the interaction between genetic predisposition and cumulative environmental exposures Collectively, the “exposome” complicates the risk stratification process for CMD. In spite of our incomplete understanding of these interactions, ongoing research into these novel pathways holds the promise of uncovering innovative preventive and therapeutic strategies.
Despite numerous investigations into the roles of lipids, oxidative stress, and inflammation in CMDs, much of the existing literature relies on a reductionist paradigm, viewing these as independent factors or studying strictly one pathway alone, typically without regard for the complex interactions and feedback patterns inherent in disease progression. Most reviews are also unable to incorporate recent advances from systems biology, omics technologies, and translational medicine, resulting in dated or fragmented models. In contrast, this manuscript outlines an even more integrative and holistic strategy, synthesizing current molecular, cellular, and clinical data to map the bidirectional interactions between lipid metabolism, oxidative stress, and chronic inflammation. By combining knowledge of emerging concepts in immune-metabolic signaling, endothelial dysfunction, and cross-talk between organs, this research outlines a more integrated and biologically germane model of CMDs, ultimately more solidly grounded in a foundation for the identification of multi-targeted therapeutic approaches.
CARDIOMETABOLIC DISEASES (CMDs)
Overview of CMDs: The CMDs, including obesity, Insulin Resistance, hypertension, and dyslipidaemia, share common metabolic dysfunctions involving blood pressure, lipid, and glucose regulation. These conditions significantly increase the risk of heart disease, stroke, and type 2 diabetes. Driven by global trends in poor diet, physical inactivity, and urbanisation, CMD prevalence has risen rapidly. Obesity especially abdominal plays a central role in CMD pathogenesis by promoting oxidative stress, Insulin Resistance, and systemic inflammation. Environmental exposures such as air pollution and chronic stress further aggravate CMD risk by triggering inflammatory and metabolic responses12-16.
The CMDs typically present as a cluster of clinical features: Abdominal obesity, hypertension, dyslipidaemia (high triglycerides, low HDL), and insulin resistance. These are also the diagnostic criteria for metabolic syndrome17. Insulin resistance, often accompanied by hyperinsulinemia, can lead to type 2 diabetes, while lipid abnormalities increase atherosclerosis risk13. Visceral fat promotes chronic inflammation via cytokines, worsening vascular dysfunction.
|
|
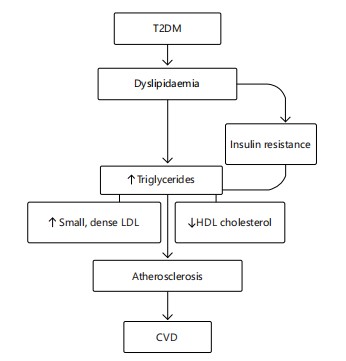
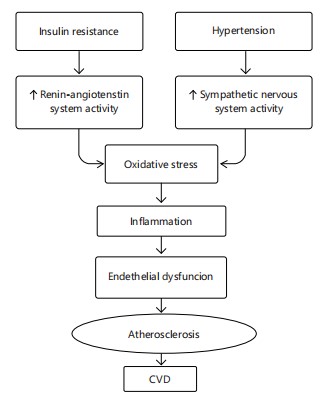
The CMD development is strongly linked to sedentary lifestyles and unhealthy diets, compounded by genetic predisposition. Management involves lifestyle interventions diet, exercise, stress reduction and pharmacologic control of metabolic parameters when necessary14 in Fig. 2 and 3.
The path of dyslipidaemia from type 2 diabetes mellitus (T2DM) to cardiovascular disease (CVD) is depicted in this graphic. Triglycerides, small dense LDL, and HDL cholesterol all rise as a result of insulin resistance and dyslipidaemia brought on by type 2 diabetes. These anomalies in lipids lead to atherosclerosis, which in turn causes CVD (Fig. 2).
The interrelated processes by which insulin resistance and hypertension lead to the development of cardiovascular disease (CVD) are depicted in this graphic. While hypertension triggers the sympathetic nervous system, insulin resistance increases the activity of the Renin-Angiotensin System (RAS). Both routes encourage oxidative stress, which sets off inflammation, a major cause of vascular damage. Endothelial dysfunction brought on by chronic inflammation affects the blood vessels capacity to dilate and control vascular tone. Atherosclerosis, which is defined by the buildup of lipid plaques within artery walls, is triggered by this malfunction. Atherosclerosis causes the arteries to thin over time, which lowers blood flow and raises the risk of cardiovascular events, including heart attacks and strokes. When combined, these overlapping pathways create a crucial connection between the pathophysiology of CVD and anomalies in metabolism and haemodynamics (Fig. 3).
LIPIDS AND CMDs
Lipid metabolism and homeostasis: Lipids are small hydrophobic or amphipathic molecules such as fatty acids (FAs), glycerolipids, phospholipids, sterols, and sphingolipids produced through thioester or isoprene unit condensations. Their biological roles depend on both individual properties and their behavior in assemblies across subcellular compartments. The FAs, synthesized mainly in the liver, adipose tissue, and lactating breast, may also be obtained from the diet, often bound to proteins like LDL15. While normal cells rely on exogenous FAs, cancer cells often upregulate endogenous synthesis. Lipids function in energy storage (e.g., triacylglycerols), membrane structure (e.g., phosphoglycerides, sterols), and signaling. They act as precursors to hormones like testosterone and progesterone, and as second messengers such as diacylglycerol and sphingosine derivatives16.
The FA synthesis begins in the mitochondria with acetyl-CoA, which enters the cytoplasm as citrate, then converts to malonyl-CoA (via ACC) and undergoes chain elongation via FASN. The FAs are also precursors to complex lipids such as phosphoglycerides and eicosanoids like prostaglandins and leukotrienes. Cholesterol, essential for membrane fluidity and signaling, is synthesized via the mevalonate pathway or taken up via LDLR. Its levels are tightly regulated to prevent toxicity, ensuring lipid homeostasis17.
Glycometabolic disorder: Glycolipid metabolic disorder (GLMD) arises from disruptions in lipid and glucose metabolism, influenced by genetic, dietary, psychological, and environmental factors. As it is closely linked to modern lifestyle patterns, GLMD is a growing global health concern, contributing to CVD and related comorbidities17. The rising prevalence of obesity poses a significant challenge for public health systems aiming to reduce chronic disease incidence and mortality. GLMD results from abnormalities in glucose and lipid synthesis, breakdown, and absorption, leading to ectopic fat accumulation. Key mechanisms include Insulin Resistance (IR), oxidative stress, metainflammation, and gut dysbiosis. These trigger metabolic conditions such as fatty liver disease, dyslipidaemia, and hyperglycemia, all of which elevate CVD risk. IR, often seen in Type 2 Diabetes Mellitus (T2DM), reflects impaired insulin action and is exacerbated by chronic inflammation in the liver, muscle, and adipose tissue, contributing to atherosclerosis and metabolic-associated fatty liver disease (MAFLD)]18.
Chronic hyperglycaemia: Chronic hyperglycemia produces harmful effects on several organ systems and contributes to the etiology of cardiovascular comorbidities by impairing glycolipid metabolism, thus initiating macrophage-mediated inflammation and causing vascular damage17. One of the key factors associated with hyperglycemia is enhanced formation of advanced glycation end products (AGEs), which have been shown to cause tissue injury through multiple mechanisms. Endogenously, AGEs are formed by oxidation of proteins, lipids, nucleotides, and sugars and by the hyperactivation of the polyol pathway in hyperglycemic conditions. Among the major representatives of AGEs are glyoxal, methylglyoxal, 3-deoxyglucosone, glycolaldehyde, and glyceraldehyde, all with pathological action either through cross-linking and modification of protein structure and function or induction of the generation of ROS and pro-inflammatory cytokines linking oxidative stress, chronic inflammation, and CVD18.
Endothelial dysfunction: Along with AGEs, endothelial dysfunction is accentuated by the overexpression of VEGF and PAI-1 molecules, which are responsible for neoangiogenesis and for a pro-thrombotic state that plays a pivotal role in the progression of CVD. The binding of AGEs to their receptor (RAGE) induces intracellular signaling pathways that enhance the secretion of pro-inflammatory cytokines like Interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), transforming growth factor-beta (TGF-β), and adhesion molecules like vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and endothelin-1 (ET-1) all of which are strongly associated with cardiovascular pathology19.
Adipose tissue dysfunction: Circling the cardiovascular system, adipose tissue is also central; its metabolic derangement encourages a shift from an anti-inflammatory (M2) to a pro-inflammatory (M1) macrophage phenotype, which in turn leads to increased release of resistin, leptin, and TNF-α and decreased adiponectin, thereby directly or indirectly affecting cardiovascular function20. Several molecular mediators bridge glycolipid metabolic disorders (GLMDs) with CVD further. For instance, angiotensin-converting enzyme 2 (ACE2), a mono-carboxypeptidase involved in amino acid intake and regulation of the Renin-Angiotensin*Aldosterone System (RAAS), is deregulated in hypertension, type 2 diabetes mellitus (DM2), obesity, and metabolic-associated fatty liver disease (MAFLD), leading to inflammation, vasoconstriction, cardiovascular injury, and oxidative stress. ATP-binding cassette transporter A1 (ABCA1) under the regulation of peroxisome proliferator-activated receptors (PPARs) has an important function in lipid and glucose homeostasis. It plays a critical role in cholesterol efflux from adipocytes and β-cell function in the pancreas, and its deficiency results in insulin resistance and an obesity-like syndrome. Glucagon-like peptide-1 (GLP-1) produced by enteroendocrine L cells from proglucagon is regulated by the gut microbiota via short-chain fatty acid signaling. It evokes insulin release, causes satiety, prevents obesity, and offers cardiovascular protection, thus linking gut health with metabolic and cardiovascular regulation21.
Toll-like receptor 4 (TLR-4): Toll-like receptor 4 (TLR-4), a key mediator of innate immunity, also modulates glucose and lipid metabolism. Its lipopolysaccharide (LPS) activation triggers cytosolic phospholipase A2 (cPLA2), having an impact on low-density and high-density lipoprotein (LDL-c and HDL-c) levels, and inhibition would potentially suppress hepatic glucose production and reduce inflammation, suggesting a possible target for the intervention of metabolic disease22. Vascular non-inflammatory molecule-1 (VNN1), a redox modulating, inflammatory, and glucose homeostasis-associated pantetheine hydrolase, is insulin resistance- and fasting-inducible, and overexpression is involved in hepatic disorders of lipid metabolism and chronic disease pathogenesis. Furthermore, elevated levels of some of the sphingolipids, specifically ceramides and sphingosine-1-phosphate (S1P), have been identified to contribute to cardiovascular diseases like atrial fibrillation, acute coronary syndromes, hypertension, myocardial ischemia, and most significantly atherosclerosis, an inflammatory condition elicited by lipid accumulation and chronic immune stimulation. These sphingolipids augment atheromatous plaque development, which is characterized by increased content of oxidized LDL (ox-LDL), TNF-α, and interleukins like IL-1β and IL-6, the latter being induced by macrophages upon plaque formation and resulting in endothelial injury and systemic inflammation. These plaques also stimulate hepatic production of C-reactive protein (CRP), the major systemic inflammation biomarker23. At the cellular level, metabolic derangements in glycolipids are directly connected to endothelial impairment, from endothelial apoptosis through chronic hyperglycemia to documenting their role in atherosclerosis pathogenesis and other cardiovascular consequences17.
OXIDATIVE STRESS
Reactive oxygen species and antioxidant defences: Reactive oxygen species (ROS) including Hydrogen Peroxide (H2O2), Hydroxyl Radicals (•OH), Superoxide (O2•), and Singlet Oxygen (¹O2) are natural by-products of metabolism. At low levels, ROS serve critical roles as secondary messengers in vascular and cardiac cells, regulating protein phosphorylation, transcription, differentiation, and immune responses.
However, excessive ROS accumulation causes damage to lipids, proteins, and DNA, contributing to a wide range of diseases such as diabetes, atherosclerosis, cardiovascular, and neurodegenerative disorders24. The ROS are produced mainly by endothelial, epithelial, and phagocytic cells (e.g., macrophages, neutrophils).
Mitochondria are primary ROS generators through complexes I and III of the electron transport chain. Complex I releases O2•−into the mitochondrial matrix, while complex III produces O2•− on both sides of the inner membrane. Enzymes such as NADPH oxidase and xanthine oxidase also produce ROS. The NADPH oxidase, activated by microbial or inflammatory stimuli, is prominent in phagocytes and vascular cells. Xanthine oxidoreductase (XOR), which exists as xanthine oxidase (XO) or dehydrogenase (XDH), produces O2•− and H2O2, especially in the liver and intestines. Nitric oxide synthase (NOS) may also generate ROS when uncoupled, often due to BH oxidation, while myeloperoxidase (MPO) further amplifies oxidative stress by converting H2O2 into reactive species25.
Oxidative stress in metabolic dysregulation: Oxidative stress results from an imbalance between excessive reactive oxygen species (ROS) production and insufficient antioxidant defenses. The ROS, including Hydrogen Peroxide (H2O2), Superoxide Anions (O2•−), and Hydroxyl Radicals (•OH), are by products of mitochondrial oxidative phosphorylation. While moderate ROS levels are essential for cellular signaling, excess ROS causes oxidative damage to proteins, lipids, and DNA. Oxidative stress both drives and reflects metabolic dysregulation, playing a key role in disorders like obesity, type 2 diabetes, and dyslipidaemia. Elevated glucose and fatty acids increase mitochondrial ROS, activating pro-inflammatory pathways (e.g., NF-κB, JNK), which impair insulin signaling and β-cell function25.
The ROS also reduces nitric oxide (NO) bioavailability, promoting endothelial dysfunction and atherosclerosis. Accumulation of oxidized lipids like oxLDL further drives vascular inflammation and cardiovascular risk. Antioxidants endogenous (e.g., glutathione) and exogenous (e.g., vitamins C,E) alongside lifestyle interventions and drugs like statins or metformin, help restore redox balance and improve metabolic health.
INFLAMMATION AND ITS METABOLIC CONSEQUENCES
Low-grade chronic inflammation: Chronic inflammation retains many acute features such as vasodilation, increased capillary permeability, and leukocyte migration. However, over time, neutrophils are replaced by macrophages, lymphocytes, and plasma cells the hallmark of chronic inflammation26. These immune cells release cytokines, enzymes, and growth factors that contribute to tissue damage, granuloma formation, and fibrosis. Tissue-resident macrophages and dendritic cells detect antigens and produce pro-inflammatory cytokines like IL-1 and TNF-α, which activate endothelial cells to express selectins and integrins, facilitating leukocyte recruitment. Once in the tissue, leukocytes are further activated and release additional inflammatory mediators.
In the acute phase, neutrophils dominate, releasing enzymes such as myeloperoxidase and matrix metalloproteinases. They phagocytose antigens and secrete TNF-α, IL-1, IL-6, and ROS. As inflammation progresses, T- and B-lymphocytes mediate responses through cytokine release and antibody production27. Platelets also contribute by releasing inflammatory mediators upon activation, highlighting their role beyond clotting in sustaining inflammation and coordinating tissue repair.
Inflammatory mechanisms in CMDs: Inflammation plays a central role in the development of Insulin Resistance (IR) through various signaling pathways. A key regulator is the transcription factor NF-κB, which is activated in obesity and promotes IR by inducing proinflammatory cytokines such as TNF-α, IL-1β, and IL-6. Inhibition of NF-κB improves insulin sensitivity, highlighting its significance in inflammation-driven IR28. Similarly, the JNK pathway, activated by ER stress and hyperglycemia, promotes IR by reducing insulin secretion and enhancing inflammatory cytokine production. The NLRP3 inflammasome is another critical pathway linking obesity, inflammation, and IR.
Obesity-induced chronic inflammation is also marked by macrophage infiltration into adipose tissue, driven by dietary fats and NF-κB signaling. Elevated hs-CRP, regulated by IL-6 and TNF-α, is frequently associated with IR and may predict type 2 diabetes risk. Systemic inflammation in obesity results from a cytokine imbalance and elevated acute-phase proteins like CRP, involving key mediators such as TNF-α, IL-6, and IL-1β29.
Chronic overnutrition exacerbates inflammation by altering adipose tissue through immune cell recruitment and proinflammatory adipokine release. This promotes fibrosis, hypoxia, and ECM remodeling. Furthermore, inflammatory dysregulation is now linked to several cardiovascular diseases, including heart failure and arrhythmias.
INTERPLAY BETWEEN LIPIDS, OXIDATIVE STRESS, AND INFLAMMATION
The CMDs are primarily driven by dysregulated lipid metabolism, which activates downstream inflammatory and oxidative stress pathways. A key event is the oxidative modification of low-density lipoprotein (LDL) into oxidized LDL (oxLDL). Macrophages engulf oxLDL via scavenger receptors, forming foam cells that initiate fatty streaks in arteries. These foam cells release pro-inflammatory cytokines such as IL-1β and TNF-α, sustaining vascular inflammation. In parallel, elevated free fatty acids and ceramides worsen insulin resistance and mitochondrial dysfunction, further fuelling metabolic dysregulation30.
Oxidative stress, resulting from excessive Reactive Oxygen Species (ROS), plays a central role in CMD progression. ROS promote lipid peroxidation, producing reactive aldehydes like malondialdehyde (MDA), which bind proteins and DNA to form advanced lipid peroxidation end products (ALEs). The ALEs act as damage-associated molecular patterns (DAMPs), activating innate immune receptors such as TLRs and RAGE. This triggers NF-κB signaling and amplifies inflammation both locally and systemically31.
The OxLDL also activates Pattern Recognition Receptors (PRRs), including TLRs and scavenger receptors, stimulating inflammatory responses and expression of cytokines like MCP-1, IL-6, and TNF-α. These contribute to endothelial dysfunction, reduce nitric oxide (NO) availability, and promote vascular stiffness and hypertension. Simultaneously, inflammation enhances hepatic lipogenesis via SREBP-1c and increases VLDL production, while inhibiting cholesterol efflux by downregulating ABCA1 and ABCG1, impairing HDL’s protective roles32.
This feedback loop of dyslipidemia, oxidative stress, and inflammation worsens insulin resistance and endothelial damage. The ROS and inflammatory cytokines disrupt insulin signaling and β-cell function, aggravating hyperglycemia and metabolic imbalance. Addressing this network through antioxidants, anti-inflammatory agents, and lifestyle interventions may offer more effective treatment. Biomarkers like MDA and oxLDL also hold potential for tracking CMD severity and therapeutic response.
THERAPEUTIC IMPLICATIONS
Lifestyle changes: The Mediterranean diet (Me-Di), rich in fruits, vegetables, legumes, whole grains, nuts, and seafood, with minimal red meat and moderate red wine intake, is widely recognized for its health benefits. It has been shown to significantly reduce cardiovascular mortality and the risk of chronic diseases such as heart disease, stroke, and neurodegenerative disorders. Adherence to the Me-Di also lowers carotid plaque burden and reduces cerebrovascular events. Regular aerobic exercise complements these effects by improving insulin sensitivity, metabolic health, and cardiovascular function, while also reducing oxidative stress and inflammation over time33.
PHARMACOLOGICAL INTERVENTIONS
Statins: Statins reduce cholesterol by inhibiting the mevalonate pathway, decreasing hepatic cholesterol synthesis, and promoting LDL receptor expression, which enhances LDL clearance. They also lower triglycerides and raise HDL levels. Beyond lipid control, statins exert anti-inflammatory and antioxidant effects, stabilize plaques, and improve endothelial function. They reduce inflammatory markers like CRP and enhance nitric oxide availability, supporting vascular health. Evidence shows statins benefit even those with normal LDL but elevated inflammation, indicating effects independent of cholesterol lowering34. Their antioxidant actions also inhibit LDL oxidation and ROS production, contributing to atheroprotection.
Antioxidants: Though evidence is mixed, vitamins C and E may benefit oxidative stress related cardiometabolic diseases like atherosclerosis, hypertension, insulin resistance, and type 2 diabetes. Vitamin C is a powerful water-soluble antioxidant that scavenges free radicals and regenerates vitamin E, while vitamin E protects lipid membranes, including LDL, from oxidative damage a key step in atherogenesis. Together, they support endothelial function and reduce inflammation. While some trials show limited or harmful effects of high-dose supplementation, others report improvements in oxidative stress and inflammation markers in at-risk individuals. Effectiveness likely varies by dose, duration, and baseline antioxidant status35.
ANTI-INFLAMMATORY AGENTS
Anti-inflammatory drugs in CMDs: Chronic low-grade inflammation is a key driver of cardiometabolic disorders like insulin resistance, obesity, and atherosclerosis. Canakinumab, an IL-1β inhibitor, has shown promise in reducing cardiovascular events in high-risk patients with elevated inflammation markers. Blocking IL-1β it reduces systemic inflammation and vascular damage. The CANTOS trial confirmed that targeting inflammation significantly reduces recurrent cardiovascular events without altering lipid levels. This supports anti-inflammatory therapy as a novel risk-reduction strategy in type 2 diabetes and metabolic syndrome, especially when traditional risk factor control is insufficient36.
PPAR agonists and kidney protection: The PPAR agonists, including TZDs and 15d-PGJ2, have shown effectiveness in managing diabetic kidney disease by inhibiting epithelial-mesenchymal transition and enhancing glucose metabolism. A meta-analysis of 2,860 diabetics demonstrated reduced urinary albumin excretion with TZD use. Beyond diabetes, PPAR agonists benefit kidney diseases like polycystic kidney disease and glomerulosclerosis. Pioglitazone improves renal function in ischemia-reperfusion injury via anti-inflammatory and antioxidant effects. Additionally, PPAR agonists like rosiglitazone reduce vascular calcification in CKD, potentially via AQP1 regulation, offering renal and vascular benefits in dialysis patients as well.
SGLT2 inhibitors in cardiometabolic therapy: The SGLT2 inhibitors prevent glucose and sodium reabsorption in the kidney, promoting glucosuria and diuresis. These agents lower blood pressure and cardiac load via natriuresis and sympathetic tone reduction. They also reduce glomerular hyperfiltration, mimic hypoxia to stimulate erythropoiesis, and offer reno and cardioprotection. Some agents also affect SGLT1, enhancing benefits37. Their anti-inflammatory, anti-apoptotic, and antioxidant effects further aid metabolic health. By boosting myocardial energy efficiency and vascular function, SGLT2 inhibitors deliver multifaceted improvements in cardiometabolic outcomes beyond glycemic control.
Plant-derived compounds and nutraceuticals: Plant compounds like resveratrol, curcumin, quercetin, and berberine target inflammation and oxidative stress in cardiometabolic disease. Resveratrol enhances insulin sensitivity via SIRT1 and AMPK, while curcumin inhibits NF-κB and reduces cytokines like TNF-α. Quercetin boosts nitric oxide bioavailability and reduces hypertension, and berberine improves insulin signaling and lowers LDL cholesterol38. These nutraceuticals influence mitochondrial function, gene expression, and gut microbiota, enhancing cardiometabolic regulation. Used with diet and exercise, they offer low-risk, holistic treatment alternatives in preventive and personalized medicine Fig. 4 and Table 1.
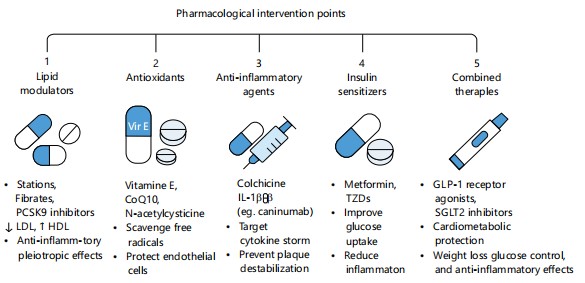
|
| Table 1: | Classification, advantages, and limitations of pharmacological interventions for CMDs | |||
| Class | Examples | Key Benefits | Limitations |
| Lipid modulators | Statins, Fibrates, PCSK9 inhibitors |
↓LDL, ↑ HDL; anti-inflammatory pleiotropic effects |
Myopathy (statins), liver enzyme elevation, cost (PCSK9 inhibitors) |
| Antioxidants | Vitamin E, CoQ10, N-acetylcysteine |
Scavenge free radicals; protect endothelium |
Mixed clinical results; may not reverse established disease |
| Anti-inflammatory agents |
Colchicine, IL-1β inhibitors (e.g. canakinumab) |
Reduce cytokine storm; stabilize atherosclerotic plaques |
GI side effects (colchicine); high cost, infection risk (IL-1β blockers) |
| Insulin sensitizers | Metformin, TZDs | Improve insulin sensitivity; anti-inflammatory; ↓ hepatic glucose production |
Weight gain (TZDs), edema, risk of heart failure (some TZDs), GI upset (metformin) |
| Combined therapies |
GLP-1 receptor agonists, SGLT2 inhibitors |
Cardiometabolic protection; weight loss; glucose & BP control; ↓ inflammation |
Injectable (GLP-1 RAs), genital infections (SGLT2is), cost |
| PPAR agonists | Pioglitazone, Fenofibrate, PPAR-α/γ dual agonists |
Regulate lipid and glucose metabolism; anti-inflammatory effects |
Fluid retention, weight gain, cardiovascular risks (agent- dependent) |
| Plant-derived compounds and nutraceuticals |
Curcumin, resveratrol, omega-3 fatty acids, berberine |
Antioxidant, anti-inflammatory, lipid- lowering properties; fewer side effects |
Variable potency, lack of standardization, and limited large-scale clinical trials |
EMERGING THERAPIES AND MOLECULAR TARGETS
Emerging therapies target molecular regulators like FGF21, a hormone that controls lipid and glucose metabolism and energy balance. Mutations in FGF21 or its receptor are linked to MASLD and hypertriglyceridemia. Clinical trials are testing FGF21 analogues for obesity and T2DM treatment. Mitochondrial dysfunction is another focus, as it underlies insulin resistance and CVD39. Gene-editing tools like CRISPR-Cas9 allow precise mitochondrial gene repair. Novel drugs aim to enhance mitochondrial biogenesis and reduce oxidative stress, while personalized medicine integrates genetic testing to improve efficacy and minimize side effects.
FUTURE TRENDS
In the future, a more profound integration of molecular mechanisms, i.e., the functions of lipids, oxidative stress, and inflammation, is needed to treat CMDs. Recent studies have illustrated how these pathways crosstalk in the etiology of such conditions as metabolic syndrome, cardiovascular disease, and type 2 diabetes. With our improved knowledge on lipid metabolism, there is increasing evidence that lipid composition, i.e., the ratio of saturated, monounsaturated, and polyunsaturated fatty acids, and amount, has a key function to play in influencing inflammatory responses. Sophisticated lipidomic profiling methods will reveal unprecedented insights into the dynamics of lipid species in tissues and in the circulatory system. By identifying new biomarkers to detect and develop cardiometabolic disease, these analyses will allow the delivery of more targeted therapies. Moreover, expansion of therapy that targets these interlinked pathways is anticipated to be promoted by further insight into the molecular interactions among lipids and cellular mechanisms engaged in inflammation and oxidative stress.
At the same time, the role of mitochondrial impairment and accumulation of reactive oxygen species (ROS) is acquiring ever greater prominence in the research on oxidative stress in cardiometabolic disease. Because the major site of cellular energy production, mitochondria, play a crucial role in cellular homeostasis. Cellular damage can result from overproduction of ROS when its activity is compromised, potentially exacerbating insulin resistance, endothelial dysfunction, and atherosclerosis. Treatment of CMDs increasingly relies on progress in mitochondrial medicine, which includes methods for enhancing mitochondrial function and alleviating oxidative stress. To restore mitochondrial viability, coming therapies can make use of gene therapies, antioxidants, and mitochondrial-targeted peptides. Moreover, mounting evidence points towards a role of the gut microbiota in regulating oxidative stress, and therefore gut-healthy interventions like probiotics and prebiotics could provide novel therapeutic avenues for decreasing inflammation and systemic oxidative damage in these conditions.
One of the most important targets for treatment is chronic low-grade inflammation, a feature of cardiometabolic disorders. The future will perhaps lie in the development of anti-inflammatory therapies targeting important inflammatory mediators like cytokines, chemokines, and pro-inflammatory transcription factors like NF-κB. Immune cell activity, specifically macrophages, which play a significant role in the etiology of insulin resistance, atherosclerosis, and other metabolic syndrome disorders, is one intriguing potential pathway to alter. Furthermore, proper means of altering pro-inflammatory gene expression may emerge through gene editing technologies like CRISPR-Cas9, which would presumably lead to highly specific treatments.
In patients with cardiovascular and metabolic diseases, concomitant application of anti-inflammatory measures with lipid-lowering agents, e.g., statins or more recently developed agents like PCSK9 inhibitors, may work synergistically to decrease the inflammation- and lipid-mediated risks. Besides, in order to effectively treat the multifactorial pathophysiology of these conditions and provide a more comprehensive, patient-tailored model of treatment, combined therapeutic strategies including lifestyle intervention, pharmacotherapy, and nutraceuticals are sure to be used to treat CMDs in the future.
CONCLUSION
Cardiometabolic diseases arise from complex interactions between oxidative stress, inflammation, and dyslipidemia, which collectively drive atherosclerosis, insulin resistance, and metabolic syndrome. Lipid imbalances such as elevated triglycerides and LDL-C exacerbate ROS production, triggering chronic inflammation and vascular damage. Future research should focus on identifying biomarkers for early diagnosis and developing combination therapies integrating antioxidants, anti-inflammatory agents, and lipid-lowering drugs to address these interconnected pathways and improve patient outcomes.
SIGNIFICANTCE STATEMENT
This study found that oxidative stress, chronic inflammation, and dysregulated lipid metabolism all play interrelated roles in the development of cardiometabolic diseases, such as type 2 diabetes, obesity, hypertension, and cardiovascular disease. This information may help develop more efficient, comprehensive approaches to early detection, prevention, and customized treatment. Important aspects of the pathophysiological interactions underlying cardiometabolic diseases that have not yet been thoroughly investigated will be revealed by this investigation. This could lead to the development of a new theory on multi-targeted, comprehensive therapeutic strategies.
REFERENCES
- Chakraborty, S., A. Verma, R. Garg, J. Singh and H. Verma, 2023. Cardiometabolic risk factors associated with type 2 diabetes mellitus: A mechanistic insight. Clin. Med. Insights: Endocrinol. Diabetes, 16.
- Alfaddagh, A., S.S. Martin, T.M. Leucker, E.D. Michos and M.J. Blaha et al., 2020. Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am. J. Preventive Cardiol., 4.
- Garbuzova, E.V., L.V. Shcherbakova, O.D. Rymar, A.D. Khudiakova, V.S. Shramko and Y.I. Ragino, 2023. Triglycerides, obesity and education status are associated with the risk of developing type 2 diabetes in young adults, cohort study. J. Pers. Med., 13.
- Khan, M.A.B., M.J. Hashim, J.K. King, R.D. Govender, H. Mustafa and J. Al Kaabi, 2020. Epidemiology of type 2 diabetes-global burden of disease and forecasted trends. J. Epidemiol. Global Health, 10: 107-111.
- Al-Jawaldeh, A. and M.M.S. Abbass, 2022. Unhealthy dietary habits and obesity: The major risk factors beyond non-communicable diseases in the Eastern Mediterranean Region. Front. Nutr., 9.
- Miller, G.E., V. Passarelli, E. Chen, I. Kloog, R.J. Wright and H. Amini, 2024. Ambient PM2.5 and specific sources increase inflammatory cytokine responses to stimulators and reduce sensitivity to inhibitors. Environ. Res., 252.
- Masenga, S.K., L.S. Kabwe, M. Chakulya and A. Kirabo, 2023. Mechanisms of oxidative stress in metabolic syndrome. Int. J. Mol. Sci., 24.
- Čolak, E. and D. Pap, 2021. The role of oxidative stress in the development of obesity and obesity-related metabolic disorders. J. Med. Biochem., 40: 1-9.
- Kurlawala, Z., P. Singh, B.G. Hill and P. Haberzettl, 2023. Fine particulate matter (PM2.5)-induced pulmonary oxidative stress contributes to changes in the plasma lipidome and liver transcriptome in mice. Toxicol. Sci., 192: 209-222.
- Kumar, V., S. Hemavathy, L.K.D. Huligowda, Mridul Umesh, P. Chakraborty, B. Thazeem and A.P. Singh, 2025. Environmental pollutants as emerging concerns for cardiac diseases: A review on their impacts on cardiac health. Biomedicines, 13.
- Mohamed, S.M., M.A. Shalaby, R.A. El-Shiekh, H.A. El-Banna, S.R. Emam and A.F. Bakr, 2023. Metabolic syndrome: Risk factors, diagnosis, pathogenesis, and management with natural approaches. Food Chem. Adv., 3.
- Monda, A., M.I. de Stefano, I. Villano, S. Allocca and M. Casillo et al., 2024. Ultra-processed food intake and increased risk of obesity: A narrative review. Foods, 13.
- Fahy, E., D. Cotter, M. Sud and S. Subramaniam, 2011. Lipid classification, structures and tools. Biochim. Biophys. Acta, Mol. Cell. Biol. Lipids, 1811: 637-647.
- Ko, S.H. and Y. Jung, 2021. Energy metabolism changes and dysregulated lipid metabolism in postmenopausal women. Nutrients, 13.
- Spaulding, S.C. and W.B. Bollag, 2022. The role of lipid second messengers in aldosterone synthesis and secretion. J. Lipid Res., 63.
- de Lima, E.P., R.C. Moretti Jr., K.T. Pomini, L.F. Laurindo and K.P. Sloan et al., 2024. Glycolipid metabolic disorders, metainflammation, oxidative stress, and cardiovascular diseases: Unraveling pathways. Biology, 13.
- Su, Y., J. Ren, J. Zhang, J. Zheng and Q. Zhang et al., 2024. Lactobacillus paracasei JY062 alleviates glucolipid metabolism disorders via the adipoinsular axis and gut microbiota. Nutrients, 16.
- Wasim, R., T. Mahmood, M.H. Siddiqui, F. Ahsan and A. Shamim et al., 2022. Aftermath of AGE-RAGE Cascade in the pathophysiology of cardiovascular ailments. Life Sci., 307.
- Arivazhagan, L., C.J. Popp, H.H. Ruiz, R.A. Wilson and M.B. Manigrasso et al., 2023. The RAGE/DIAPH1 axis: Mediator of obesity and proposed biomarker of human cardiometabolic disease. Cardiovasc. Res., 119: 2813-2824.
- MacLean, M., J. Derk, H.H. Ruiz, J.K. Juranek, R. Ramasamy and A.M. Schmidt, 2019. The Receptor for Advanced Glycation End Products (RAGE) and DIAPH1: Implications for vascular and neuroinflammatory dysfunction in disorders of the central nervous system. Neurochem. Int., 126: 154-164.
- Greiner, T.U., A. Koh, E. Peris, M. Bergentall and M.E.V. Johansson et al., 2024. GLP-1R signaling modulates colonic energy metabolism, goblet cell number and survival in the absence of gut microbiota. Mol. Metab., 83.
- Qin, W., M. Kang, C. Li, W. Zheng and Q. Guo, 2023. VNN1 overexpression in pancreatic cancer cells inhibits paraneoplastic islet function by increasing oxidative stress and inducing β-cell dedifferentiation. Oncol. Rep., 49.
- Düsing, P., N.N. Heinrich, B. Al-Kassou, K. Gutbrod and P. Dörmann et al., 2023. Analysis of circulating ceramides and hexosylceramides in patients with coronary artery disease and type II diabetes mellitus. BMC Cardiovasc. Disord., 23.
- Taniyama, Y. and K.K. Griendling, 2003. Reactive oxygen species in the vasculature: Molecular and cellular mechanisms. Hypertension, 42: 1075-1081.
- Kozlov, A.V., S. Javadov and N. Sommer, 2024. Cellular ROS and antioxidants: Physiological and pathological role. Antioxidants, 13.
- Mosalmanzadeh, N. and B.D. Pence, 2024. Oxidized low-density lipoprotein and its role in immunometabolism. Int. J. Mol. Sci., 25.
- Milenkovic, V.M., E.H. Stanton, C. Nothdurfter, R. Rupprecht and C.H. Wetzel, 2019. The role of chemokines in the pathophysiology of major depressive disorder. Int. J. Mol. Sci., 20.
- Needham, E.J., A. Helmy, E.R. Zanier, J.L. Jones, A.J. Coles and D.K. Menon, 2019. The immunological response to traumatic brain injury. J. Neuroimmunol., 332: 112-125.
- Sakurai, H., S. Suzuki, N. Kawasaki, H. Nakano, T. Okazaki et al., 2003. Tumor necrosis factor-α-induced IKK phosphorylation of NF-κB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J. Biol. Chem., 278: 36916-36923.
- Scheau, C., L.G. Mihai, I.A. Bădărău and C. Căruntu, 2020. Emerging applications of some important natural compounds in the field of oncology. Farmacia, 68: 984-991.
- de Almeida, A.J.P.O., J.C.P.L. de Oliveira, L.V. da Silva Pontes, J.F. de Souza Júnior and T.A.F. Gonçalves et al., 2022. ROS: Basic concepts, sources, cellular signaling, and its implications in aging pathways. Oxid. Med. Cell. Longevity, 2022.
- Caturano, A., M. Rocco, G. Tagliaferri, A. Piacevole and D. Nilo et al., 2025. Oxidative stress and cardiovascular complications in type 2 diabetes: From pathophysiology to lifestyle modifications. Antioxidants, 14.
- Tauil, R.B., P.T. Golono, E.P. de Lima, R. de Alvares Goulart and E.L. Guiguer et al., 2024. Metabolic-associated fatty liver disease: The influence of oxidative stress, inflammation, mitochondrial dysfunctions, and the role of polyphenols. Pharmaceuticals, 17.
- Gomez-Cabrera, M.C., E. Domenech and J. Vina, 2008. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radical Biol. Med., 44: 126-131.
- Wiklund, O., L. Mattsson-Hultén, E. Hurt-Camejo and J. Oscarsson, 2002. Effects of simvastatin and atorvastatin on inflammation markers in plasma. J. Intern. Med., 251: 338-347.
- Daiber, A. and S. Chlopicki, 2020. Revisiting pharmacology of oxidative stress and endothelial dysfunction in cardiovascular disease: Evidence for redox-based therapies. Free Radical Biol. Med., 157: 15-37.
- Gyimesi, G., J. Pujol-Giménez, Y. Kanai and M.A. Hediger, 2020. Sodium-coupled glucose transport, the SLC5 family, and therapeutically relevant inhibitors: From molecular discovery to clinical application. Pflügers Arch. Eur. J. Physiol., 472: 1177-1206.
- Yi, H., H. Peng, X. Wu, X. Xu and T. Kuang et al., 2021. The therapeutic effects and mechanisms of quercetin on metabolic diseases: Pharmacological data and clinical evidence. Oxid. Med. Cell. Longevity, 2021.
- Tan, H., T. Yue, Z. Chen, W. Wu, S. Xu and J. Weng, 2023. Targeting FGF21 in cardiovascular and metabolic diseases: From mechanism to medicine. Int. J. Biol. Sci., 19: 66-88.
How to Cite this paper?
APA-7 Style
Samson,
A.O., Dimeji,
I.Y., Aduke,
S.O., Murtala,
N. (2025). Lipids, Oxidative Stress, Inflammation, and Cardiometabolic Disorders: An Integrated Approach. Trends in Medical Research, 20(1), 50-63. https://doi.org/10.3923/tmr.2025.50.63
ACS Style
Samson,
A.O.; Dimeji,
I.Y.; Aduke,
S.O.; Murtala,
N. Lipids, Oxidative Stress, Inflammation, and Cardiometabolic Disorders: An Integrated Approach. Trends Med. Res 2025, 20, 50-63. https://doi.org/10.3923/tmr.2025.50.63
AMA Style
Samson
AO, Dimeji
IY, Aduke
SO, Murtala
N. Lipids, Oxidative Stress, Inflammation, and Cardiometabolic Disorders: An Integrated Approach. Trends in Medical Research. 2025; 20(1): 50-63. https://doi.org/10.3923/tmr.2025.50.63
Chicago/Turabian Style
Samson, Aina, Olawale, Igbayilola Yusuff Dimeji, Saliu Oluremi Aduke, and Ngabea Murtala.
2025. "Lipids, Oxidative Stress, Inflammation, and Cardiometabolic Disorders: An Integrated Approach" Trends in Medical Research 20, no. 1: 50-63. https://doi.org/10.3923/tmr.2025.50.63

This work is licensed under a Creative Commons Attribution 4.0 International License.