Use of PCR Technique in Early Diagnosis of HIV Exposed Infants in Abuja, Nigeria
| Received 06 Apr, 2024 |
Accepted 09 May, 2024 |
Published 10 May, 2024 |
Background and Objective: Human Immunodeficiency Virus (HIV) disease is a major global health problem and is associated with significant morbidity and mortality. The study aimed at carrying out early diagnosis of HIV infection using polymerase chain reaction technique on HIV-exposed infants to establish the incidence of infection and risk factors. The PCR is a sensitive and specific tool for the detection of HIV in infants exposed to the virus and is essential for appropriate management and prevention of HIV transmission. Materials and Methods: This study involved 163 children born to HIV-infected mothers at the University of Abuja Teaching Hospital. Records included antiretroviral drug usage, birth date, sex, delivery mode, breastfeeding, age and PCR DNA test results. Blood was collected from the heel, big toe and left ring finger. Results: The study found that 43.3% of HIV-exposed infants tested positive, with 68.7% negative. The majority were on artificial milk, with 46.6% on breast milk. The study also found a significant difference in positive results, with 24 out of 72 males and 30 out of 91 females testing positive. Conclusion: This research examines the potential for using PCR to improve the early diagnosis of HIV in infants. It also explains how PCR techniques are used in the diagnosis and management of HIV in infants and discusses the challenges associated with using PCR in HIV-exposed infants.
| Copyright © 2024 Fredrick et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Human Immunodeficiency Virus (HIV) disease is a major global health problem and is associated with significant morbidity and mortality. Recent estimates indicate about 33 million people living with HIV infection; 15.5 million are women and 2 million are children under age of 15. In Nigeria, it was estimated that 220,000 people are living with HIV. It is the preferred laboratory technique for detecting HIV infection in infants born to mothers who test positive for the virus1,2. A PCR test administered at six weeks of age has been shown to be more advantageous than an algorithm that employs an HIV enzyme immune assay (EIA) administered at twelve months of age. Early identification of HIV infection in children enables prompt antiretroviral and preventative treatment management, which can avert serious infection and associated neurological and immunological problems. The most common methods for diagnosing HIV infection in infants are serological HIV-1 diagnostic assays, which can be challenging to interpret because HIV antibodies can remain for up to 18 months1.
Alternatively, the World Health Organisation (WHO) and the United Nations International Children Emergency Fund (UNICEF) have advised nations to make virology testing available to newborns who have been exposed, with the Polymerase Chain Reaction (PCR) technology being the recommended approach3. Finding the HIV-positive child before clinical illness manifests itself in the first few months of life is the main objective of early newborn diagnosis. Eliminating infection in newborns is not the aim. In an infant with a rapidly developing HIV illness, the diagnosis should be made early enough to allow for the start of anti-retroviral therapy and intervention to prevent pediatric mortality and morbidity. Reports of HIV transmission from mother to child (TMTC) vary by country and range from 15% to over 40% in the absence of antiretroviral therapy. Transmission by breast milk can happen postpartum, during labor and delivery, or in utero. The majority of the transmission happens during labor and late in pregnancy. Viral factors, such as viral load, genotype and phenotype strain diversity and viral resistance; maternal factors, such as clinical and immunological status, nutritional status and behavioural factors, like drug use and sexual practice; and obstetric factors, such as length of ruptured membranes, are all linked to an increased risk of transmission. Infant factors are primarily associated with the higher risk of transmission through nursing, as well as the mode of birth and intrapartum hemorrhage. Three major cohort studies undertaken in Côte d'Ivoire, South Africa and Zimbabwe have revealed that exclusive breastfeeding for up to six months was related to a three to four-fold decreased risk of transmission of HIV as compared to non-exclusive breastfeeding4,5.
There is a dearth of information on mother-to-child transmission of HIV in Nigeria in relation to the risk factors using PCR techniques to diagnose infection during the early stage of childbirth. The study aimed at carrying out early diagnosis of HIV infection using polymerase chain reaction technique on HIV-exposed infants to establish the incidence of infection and risk factors, to determine the prevalence of HIV infection in infants from HIV-infected mothers and to determine if the outcome of infection is affected by the combination of measures taken during Prevention of Mother to Child Transmission (PMTCT) care.
MATERIALS AND METHODS
Study area: This project was carried out at University of Abuja Teaching Hospital (UATH) Gwagwalada, Abuja. The study was carried from September 2011 to November 2011.
Sample collection and size
Inclusion criteria:
| • | Infants born of HIV-infected mothers within 18 months of age, whose mothers are attending PMTCT in UATH | |
| • | Infant born of HIV infected mothers on ART and not on ART who are within the age of 6 weeks, 18 months in UATH | |
| • | Infants born of HIV infected mothers attending UATH PMTCT without complication |
Exclusion:
| • | Infant born of HIV-infected mothers attending UATH PMTCT with complications | |
| • | Infants born of HIV infected mothers with or without complications above the age of 18 months | |
| • | Infants born of HIV-infected mothers who are not attending PMTCT in UATH |
Ethical approval: Ethical clearance was sought by applying to the committee in charge of ethical clearance of the UATH and was obtained.
Method
Study group: A total number of 163 children born of HIV-infected mothers attending University of Abuja Teaching Hospital, Gwagwalada Abuja were recruited for this study. Records were obtained of the type and duration of antiretroviral drug/measures taken at PMTCT care, date of birth sex of the child, mode of delivery, breastfeeding, age of child at the time of testing, the result of the PCR DNA test result of the child and relating to the health of the subjects.
Collection of blood and sample treatment
Site of blood collection: Infant from 6 weeks to 4 months; blood was collected from the heel. Infant from 4 months to 6 months; blood was collected from the big toe. The Infant`s 10 months above; the left ring finger was the site of blood collection.
Procedure: The site of blood collection was cleaned and allowed to dry. The selected part was stuck with 2 mm lancet, the filter paper was punched gently against the large drop of blood and allowed to fill the circles on the paper. The punched site was cleaned and gently pressed with cotton wool until the blood stopped. The DBS paper was air-dried on the drying rack for at least 3 hrs without stacking them.
Once dry, the individual DBS card was wrapped with glassine paper so that DBS cards would not have direct contact with each other the sealable humidity-proof plastic bag with 10 desiccants (drying agent) and humidity card bag. The bag was gently pressed to remove most of the air before sealing. Proper labeling was done before the analysis was carried out.
Principle and procedure of PCR: The principle and the method applied for the sampled analysis were according to the manufacturer’s instruction.
Sample analysis: The processing of the samples collected on dry blot spot (DBS) starts from the extraction of DNA.
RESULTS
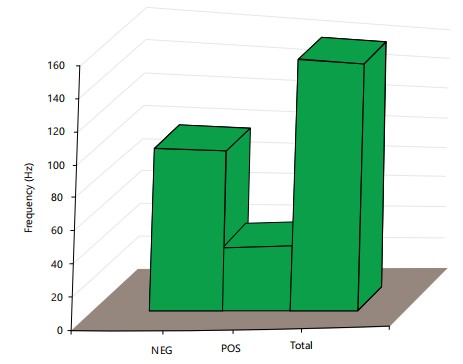
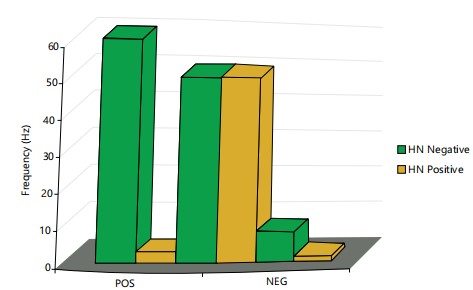
A total number of 163 HIV-exposed infants were recruited 54 (33%) tested positive, out of the 54 that tested positive only 2 received ARV and tested positive while 52 (96.3%) of the 54 HIV-exposed infants that tested positive were not on ARV in (Fig. 1). Both subjects were on AZT/NVP. The remaining 109 (67%) of HIV-exposed infants tested negative. Figure 2 expresses HIV status of infants born to HIV-infected mothers in relation to ART use.
|
|
| Table 1: | Outcome of infection in HIV pregnancies in relation to type of ART administered to neonate crosstab count | |||
| HIV status of infants | |||
| PMTCT traetment to neonate | NEG | POS | Total |
| AZT/3TC | 5 | 0 | 5 |
| 3TC/NVP/D4T | 3 | 0 | 3 |
| 3TC/ZDV/NUP | 1 | 0 | 1 |
| 3TC/ZDV/NVP | 1 | 0 | 1 |
| D4T/3TC/NVP | 1 | 0 | 1 |
| HAART | 21 | 4 | 25 |
| None | 43 | 45 | 88 |
| NUP | 1 | 0 | 1 |
| NUP/ZDV | 1 | 0 | 1 |
| NVP | 5 | 3 | 8 |
| NVP and ZDV | 1 | 0 | 1 |
| NVP/3TC/ZDV | 4 | 0 | 4 |
| NVP/AZT | 1 | 0 | 1 |
| NVP/ZDV | 9 | 0 | 9 |
| NVP/ZDV/3TC | 4 | 0 | 4 |
| STC/ZDV/NVP | 0 | 1 | 1 |
| ZDV/3TC | 1 | 0 | 1 |
| ZDV/NVP | 1 | 0 | 1 |
| ZDV/NVP/3KT | 1 | 1 | 2 |
| ZDV/NVP/3TC | 4 | 0 | 4 |
| Total | 10954 | 163 | |
Table 1 shows the outcome of infection in HIV pregnancies in relation to type of ART administered to neonate crosstab count. Table 2 expresses the outcome of infection in HIV pregnancies in relation to type of ART administered during labor and Table 3 shows the result of HIV status of infants born of HIV-infected mothers.
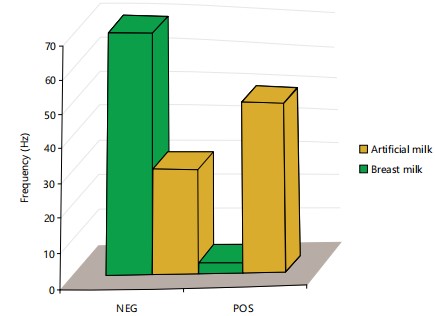
Mothers of the HIV-exposed infants numbering 120 did not use ART during labor, 52 (43.3%) of their infants tested positive, 68 (56.7%) tested negative while 43 mothers of the HIV-exposed infants on ART all their infants tested negative indicating a significant difference between those taking ART and those not placed on ART. As 76 (46.6%) HIV-exposed infants are on artificial milk while 87 (53.4%) received breast milk. As 3 of the HIV-exposed infants on artificial milk tested positive while 51 of them on breast milk were positive (Fig. 3). The differences observed were statistically significant, as a higher number of breast milk tested positive.
|
| Table 2: | Outcome of infection in HIV pregnancies in relation to type of ART administered during labour | |||
| HIV status of infants | |||
| Treatment in labour | NEG | POS | Total |
| 3TC/DAT/NVP | 10 | 10 | 10 |
| 3TC/NVP/D4T | 1 | 0 | 1 |
| 3TC/NVP/ZDV | 1 | 0 | 1 |
| AZT/NVP/ZDV | 2 | 0 | 2 |
| 1 | 0 | 1 | |
| AZT/NVP | 6 | 0 | 6 |
| HAART | 1 | 0 | 2 |
| None | 9 | 0 | 9 |
| NVP/ZDV | 68 | 52 | 120 |
| NUP | 1 | 0 | 1 |
| NVP | 4 | 2 | 6 |
| NVP/3TC | 1 | 0 | 1 |
| ZDV/NVP/3TC | 4 | 0 | 4 |
| Total | 109 | 54 | 163 |
| Table 3: | HIV status of infants born of HIV-infected mothers | |||
| HIV status of infants | |||
| Sex of the baby | NEG | POS | Total |
| Female | 48 (67%) | 24 (33%) | 72 |
| Male | 61 (67%) | 30 (33%) | 91 |
| Total | 109 | 54 | 163 |
As 24 out of 72 males and 30 of 91 females tested positive. This difference was statistically significant.
DISCUSSION
A total number of 163 HIV-exposed infants were recruited 54 (33%) tested positive, out of the 54 that tested positive only 2 received ARV and tested positive while 52 (96.3%) of the 54 HIV-exposed infants tested positive. The HIV infection has been reported to affect pregnancy outcomes or complications in the developed world3-5. This was in contrast to studies conducted in Africa5,6 and the present study in which the prevalence of infection is unacceptably high, considering the availability of intervention to prevent mother to child transmission of HIV. A total number of 54 (33%) HIV-exposed infants tested positive and 109 (67%) negative (Fig. 1).
The result of this study was consistent with the view of7. In these studies, reported rates of transmission of HIV from mother to mother to child range from around 15-25% in Europe and the USA from 25-40% in Africa and Asia. Only 2 subjects out of a total of 54 who received Art tested positive (Fig. 2). Both subjects were on AZT/NVP. This is not a surprising outcome since both Zidovudine and Nevirapine are class C drugs whose safety in pregnancy has not been determined by the FDA report on classification of antiretroviral drugs for use in pregnancy.
As 52 of the 54 subjects, who tested positive in this study, representing 96% were not on ART. There was a significant difference between subjects taking ART and those not placed on ART. Several studies of Dabis et al.8, have reported the effectiveness of combination ART in the prevention of top mother to child transmission of HIV. The high incidence of infection in the study population who were not administered, ART confirms the need for its use. Current guidelines for HIV medication therapy include using at least two agents, with the possibility of adding a protease inhibitor.
In labor, 120 out of all the infants exposed to HIV did not use antiretroviral therapy. Of these, 52 (43%) tested positive and 68 (57%) tested negative. Determining the risk of transmission intrapartum and through breastfeeding may also be significantly influenced by the local viral load in breast milk and cervical-vaginal secretions9,10.
This may account for the pattern observed in this study in which 76 (46.6%) subjects were placed on artificial milk, while 87 (53.4%) received breastfeeding milk. As 3 of the subjects were on artificial milk, while 87 (53.4%) received breastfeeding milk. As 3 of the HIV-exposed infants on artificial milk tested positive while 51 of the HIV-exposed infants on statistically significant. Infection through breastfeeding has been associated with a lack of IGm and IgAin breast milk11. As 24 out of 72 males and 30 out of 91 females tested pregnant and the sex of the HIV-exposed infants. The HIV status was associated with spontaneous vaginal delivery through spontaneous vaginal delivered (SVD) were positive. Elective lower recorded 100% HIV-negative infection, although only 26 and 6 HIV-exposed infants were tested respectively. These differences were statistically significant (p<0.05).
The study recommended that heart to heart centre for counseling and voluntary HIV testing should be made available in all the hospitals and clinics to know your HIV status. Provision of PCR equipment and reagents in all hospitals and clinics being attended by pregnant mothers (ante-natal clinics). Training of personnel involved in equipment handling and diagnosis of HIV infection especially in neonates including sponsorship for local and international conferences on HIV in order to gain more knowledge on the job. The HIV infected mother should report early to PMCT centre to prevent the transmission of infection to their children. Further research into the subject, especially aspects not covered by this study is encouraged.
CONCLUSION
In this study, the prevalence of HIV infection of infants born by mothers diagnosed positive for HIV was 33% there was a significant difference between infants administered ART and those who did not use ART. The mode of breastfeeding was a risk factor, as the incidence of HIV infection was found to be high in infants breastfed compared to those placed on artificial milk. There was no significant correlation between the sex of the infants and the outcome of pregnancy as related to infection rates. The mode of delivery, breastfeeding and ART use for mothers, neonates and during labor significantly. Influence reduced the rate of infection of infants born of HIV and the efficacy was high since no discrepancies were noted in the subsequent testing. Conclusively, the outcome of infant HIV infection is affected by the combination of measures taken during PMTCT care and the PCR technique is sufficiently sensitive for testing and monitoring HIV infection.
SIGNIFICANCE STATEMENT
Human Immunodeficiency Virus (HIV) disease is a major global health problem and is associated with significant morbidity and mortality. Recent estimates indicate about 33 million people living with HIV infection; 15.5 million are women and 2 million are children under age of 15. The study aimed at carrying out early diagnosis of HIV infection using polymerase chain reaction technique on HIV-exposed infants to establish the incidence of infection and risk factors, to determine the prevalence of HIV infection in infants from HIV-infected mothers and to determine if the outcome of infection is affected by the combination of measures taken during Prevention of Mother to Child Transmission (PMTCT) care.
REFERENCES
- Chantry, C.J., E.R. Cooper, S.I. Pelton, C. Zorilla, G.V. Hillyer and C. Diaz, 1995. Seroreversion in human immunodeficiency virus-exposed but uninfected infants. Pediatr. Infect. Dis. J., 14: 382-387.
- Bergstrom, S., A. Sonnerborg, N.B. Osman and A. Libombo, 1995. HIV infection and maternal outcome of pregnancy in Mozambican women: A case-control study. Sexually Transmitted Infect., 71: 323-324.
- Temmerman, M., A.O. Nyong'o, J. Bwayo, K. Fransen, M. Coppens and P. Piot, 1995. Risk factors for mother-to-child transmission of human immunodeficiency virus-1 infection. Am. J. Obstet. Gynecol., 172: 700-705.
- Brocklehurst, P. and R. French, 1998. The association between maternal HIV infection and perinatal outcome: A systematic review of the literature and meta-analysis. Br. J. Obstet. Gynaecol., 105: 836-848.
- Hira, S.K., U.G. Mangrola, C. Mwale, C. Chintu, G. Tembo, W.E. Brady and P.L. Perine, 1990. Apparent vertical transmission of human immunodeficiency virus type 1 by breast-feeding in Zambia. J. Pediatr., 117: 421-424.
- Minkoff, H., D.N. Burns, S. Landesman, J. Youchah and J.J. Goedert et al., 1995. The relationship of the duration of ruptured membranes to vertical transmission of human immunodeficiency virus. Am. J. Obstet. Gynecol., 173: 585-589.
- Newell, M.L., 1995. Vertical transmission of HIV-1: Risks and prevention. J. Hosp. Infect., 30: 191-196.
- Dabis, F., L. Mandelbrot, P. Msellati and P. van de Perre, 1995. Zidovudine to decrease mother-to-child transmission of HIV-1: Is it good for developing countries? AIDS, 9: 204-206.
- John, G.C., R.W. Nduati, D. Mbori-Ngacha, J. Overbaugh and M. Welch et al., 1997. Genital shedding of human immunodeficiency virus type 1 DNA during pregnancy: Association with immunosuppression, abnormal cervical or vaginal discharge, and severe vitamin A deficiency. J. Infect. Dis., 175: 57-62.
- Clemetson, D.B.A., G.B. Moss, D.M. Willerford, M. Hensel and W. Emonyi et al., 1993. Detection of HIV DNA in cervical and vaginal secretions: Prevalence and correlates among women in Nairobi, Kenya. JAMA, 269: 2860-2864.
- van de Perre, P., N. Meda, M. Cartoux, L. Mandelbrot, V. Leroy, F. Dabis and R. Salamon, 2009. Zidovudine and breast-feeding. AIDS Patient Care STDs, 11: 4-5.
How to Cite this paper?
APA-7 Style
Fredrick,
C.C., Dennis,
A., Dansura,
M.L. (2024). Use of PCR Technique in Early Diagnosis of HIV Exposed Infants in Abuja, Nigeria. Trends in Medical Research, 19(1), 72-78. https://doi.org/10.3923/tmr.2024.72.78
ACS Style
Fredrick,
C.C.; Dennis,
A.; Dansura,
M.L. Use of PCR Technique in Early Diagnosis of HIV Exposed Infants in Abuja, Nigeria. Trends Med. Res 2024, 19, 72-78. https://doi.org/10.3923/tmr.2024.72.78
AMA Style
Fredrick
CC, Dennis
A, Dansura
ML. Use of PCR Technique in Early Diagnosis of HIV Exposed Infants in Abuja, Nigeria. Trends in Medical Research. 2024; 19(1): 72-78. https://doi.org/10.3923/tmr.2024.72.78
Chicago/Turabian Style
Fredrick, Christy, Chinyere, Amaechi Dennis, and Mangpin Leviticus Dansura.
2024. "Use of PCR Technique in Early Diagnosis of HIV Exposed Infants in Abuja, Nigeria" Trends in Medical Research 19, no. 1: 72-78. https://doi.org/10.3923/tmr.2024.72.78

This work is licensed under a Creative Commons Attribution 4.0 International License.