Association Between Hyperglycemia and Epilepsy: From Clinical Implication to Therapeutics
| Received 15 Apr, 2024 |
Accepted 07 Jun, 2024 |
Published 10 Jul, 2024 |
Epilepsy and hyperglycemia are significant pathologies that are increasingly recognized to have an interlinked relationship. Epidemiological data highlights the growing prevalence of concurrent diabetes mellitus (DM) and epilepsy, emphasizing the need for tailored management strategies. Therefore, this study explores the complex association between hyperglycemia (particularly DM) and epilepsy. The Google Scholar, PubMed (NIH), CDC and WHO databases were searched using an advanced search feature manually through the second week of April 2024 for Journal articles, books and book chapters were manually searched under all languages without filter restrictions. The keywords used for the search are given below. This review delves into the pathogenesis of hyperglycemia-induced changes in brain function, including alterations in neurotransmitter activity, electrolyte imbalances and effects on neuronal excitability. The coexistence of DM and epilepsy presents clinical challenges, as certain antiepileptic medications can impact glycemic control and vice versa. The review examines the epidemiology of these coexisting conditions, focusing on risk factors, age of onset and potential comorbidities. The intricate pathways linking hyperglycemia to seizures, including the role of glutamate and ionotropic receptors, are dissected, shedding light on the mechanisms behind epileptic episodes in DM patients. This study highlights the vulnerability to seizures in T1DM patients due to cerebrovascular dysfunctions and altered seizure thresholds in specific brain structures. Furthermore, the management approaches for these comorbid conditions are discussed, encompassing non-pharmacological interventions such as lifestyle modifications and pharmacological treatments targeting both epilepsy and hyperglycemia. This comprehensive review synthesizes current knowledge on the intricate relationship between hyperglycemia and epilepsy in DM patients. The elucidation of underlying mechanisms and the development of effective management strategies are crucial to improving clinical outcomes in this growing patient population. Further research is needed to understand the intricacies of the connection and to improve treatment procedures for those who have DM and epilepsy.
| Copyright © 2024 Patel et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Epilepsy is a chronic neurological condition characterized by recurrent seizures. It can occur in all ages of the population and has been known to have significant impacts on the mental and physical well-being of patients1. Hyperglycemia, on the other hand, is a condition characterized by high blood sugar levels2 and is a hallmark of diabetes mellitus (DM)3, a metabolic condition with a range of effects, including cognitive deficits, cardiovascular disease and kidney disease4,5. Hyperglycemia often induces cellular damage and death deleterious enough that it cannot be readily repaired by the body’s natural mechanisms6. Recent evidence has established a strong association between hyperglycemia and seizure episodes in patients with DM, which is deemed to be more than just coincidental. In fact, approximately 25% of patients with diabetes are expected to experience seizures2. Further, diabetic patients who experience Diabetic Ketoacidosis (DKA), a hyperglycemic emergency characterized by increased blood glucose levels, elevated urinary/blood ketoacids and high anion gap metabolic acidosis7, also experience seizures more frequently2. Series of case reports have affirmed the circadian instability associated with hyperglycemia that majorly affects the sleep-wake cycle in patients with DM8-11. It was also reported that sleep disorders are significantly more persistent in patients with DM as compared to those without diabetes12-15. There has long been a strong belief that the hyperarousal idea of neurons is a concerning etiological element in insomnia and there has been thorough scientific investigation into both the source and impact of this conception16-19. Similar to this, naturaldevelopment and function are impacted and damaged by the circadian contributions to and reactions from persistent hyperarousal. To counterbalance neuronal firing, neural plasticity in dysregulated circumstances allocates resources to hyper-activated neuronal networks, compensates for neural recovery and maintains intrinsic system stability11,20.
About 30% of individuals with Temporal Lobe Epilepsy (TLE) are resistant to standard antiepileptic medications, despite the fact that some antiepileptics have been shown to be effective in stopping or reducing the frequency of epileptic episodes21. In addition to conventional antiepileptic medication, targeting and controlling glucose levels in DM patients may offer a cutting-edge and effective alternative treatment approach for treating seizures11. A meta-analysis performed to explore the association between hyperglycemia and epilepsy provides recommendations for managing these coexisting conditions.
Epidemiology: The DM and epilepsy are two chronic conditions affecting millions of individuals worldwide. With regards to diabetes mellitus/insipidus, it is estimated that approximately 537 million populations are afflicted worldwide, with diabetes mellitus being the most predominant form22. The prevalence of DM is increasing at an alarming rate, with an estimated 1 in 11 adults by 2040. On the other hand, epilepsy is estimated to affect approximately 50 million patients globally23,24.
The coexistence of diabetes mellitus and epilepsy is becoming increasingly common and the epidemiology of this population is of growing interest25. Studies have shown that people with diabetes mellitus have an elevated risk of developing epilepsy and conversely, people with epilepsy have a higher risk of developing diabetes mellitus26. The prevalence of both conditions is higher in low- and middle-income countries. The age of onset of diabetes mellitus and epilepsy is similar, with a higher incidence in children/adolescents and older adults27. There is also an increased prevalence of comorbidities such as hypertension and other cardiovascular disease in patients with such coexistence28. Management of patients with concurrent diabetes mellitus and epilepsy can be challenging, as certain antiepileptic medications can worsen glycemic control29-31, while some antihyperglycemics can attenuate seizure threshold32-34. Understanding the epidemiology of patients with concurrent diabetes mellitus and epilepsy is essential for optimal management strategies and clinical outcomes in this patient population35.
Pathogenesis of hyperglycemia and epilepsy: Though the precise pathogenesis of seizures associated with DM remains undetermined, several known mechanisms do exist that support the hypothesis that hyperglycemia can lead to changes in brain function, including alterations in electrical activity, which can trigger seizure episodes in patients with epilepsy36. Glutamate, an excitatory neurotransmitter, is more abundant in the central nervous system37. Ionotropic glutamate receptors include N-methyl-d-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), kainite and delta receptors classified based on sequence similarity and ligand specificities38.
Over-activation of N-methyl-D-aspartate (NMDA) receptors, which are ionotropic glutamate receptors, impairs neurons in the nervous system. Although it has been suggested that NMDA receptor activation aids in the progression of diabetes mellitus, little is known about the impact of excessive long-term NMDAR activity on β-cells, particularly with episodes of hyperglycemia. One study established that an increase in glutamate, an excitatory neurotransmitter, may accelerate the malfunction and apoptosis caused by hyperglycemia in β-cells, contributing to the progression of diabetes mellitus39. Endogenous glutamate exacerbates the high-glucose-induced dysfunction of β-cells in diabetes by over-activating NMDA receptors on β-cells, which in turn activates NF-κB and NLRP3 inflammasomes. Early alterations in AMPA receptor subunits, primarily in the GluA2 subunit, are induced by high glucose concentration in retinal neuron cells. In contrast, it appears that the expression of AMPA receptors in hippocampus neurons is unaffected by high glucose levels, indicating that retinal cells are more vulnerable to the stress brought on by high glucose levels than hippocampal neurons.
Thirty to forty percent of cerebral synapses implement GABA, the brain's primary inhibitory neurotransmitter, to promote inhibition and, by limiting nerve transmission, to lower neuronal excitability40. The GABA is synthesized when glutamic acid decarboxylase (GAD) transforms L-glutamate. GABAergic inhibition of the dorsal motor nucleus of the vagus (DMV) neurons changes substantially after chronic hyperglycemia/hypo-insulinemia, which may play a role in the autonomic dysfunction associated with diabetes38. The vagus nerve, a cranial nerve that is essential in controlling the parasympathetic nervous system, has DMV as a key component. The DMV is in possession of regulating the parasympathetic nervous system's efferent (motor) impulses that are carried to the heart, lungs, gastrointestinal tract and other visceral tissues41. The effects of DMV inhibition on autonomic systems and physiological functioning can be profound25. The degree and length of inhibition and the conditions in which it transpires may all affect the specific effects, as with any intervention affecting neural networks. According to one case study, people with diabetes mellitus exhibit alterations to their GABAergic neurotransmitter system, which are linked to deficitcognitive performance. The principal findings indicated that those with diabetes mellitus, higher fasting blood glucose levels and higher glycated hemoglobin levels, as well as those with worse cognitive function, all had greater GABA concentrations. Mechanical hyperalgesia and depolarization of the resting membrane potential of primary nociceptive neurons are triggered by hyperglycemia39. Hyperglycemia delays terminal depolarization in the ischemic core and facilitates faster repolarization in severely malperfused penumbral tissue after spreading depression because it increases the availability of energy substrates in this state.
Alongside the neurotransmitters and receptor stimulation, hyperglycemia is also linked to electrolyte imbalance, especially sodium (hyponatremia) and calcium (hypercalcemia), that are linked with augmented morbidity and mortality42,43. Patients with diabetes often experience hyponatremia, or reduced blood sodium levels, which can be linked to a variety of underlying pathogenetic causes41,44,45. Patients with poorly controlled diabetes mellitus have varying serum sodium levels because these levels can occasionally be attributed to hyponatremia caused by hyperglycemia, hypotonic losses caused by osmotic diuresis, which tends to raise serum sodium levels and hypovolemia-induced decrease in serum sodium levels46,47. Reduced risk of adverse effects such as falling, impaired gait and cognitive impairment is linked to even the smallest drops in blood salt levels48. Hyponatremia in DM patients is also attributed to the use of thiazide diuretics49,50 and first generation sulfonylureas (tolbutamide and chlorpropamide)51,52 which leads tosodium loss in urine27. Though the incidence of primary hyperparathyroidism in DM is less than 1%, elevation of the serum calcium level is closely associated with a variety of conditions linked to hyperparathyroidism53,54. Long-term insulin resistance or insufficiency can cause hyperparathyroidism,which can worsen glycemic dysregulation in the development of diabetes mellitus or cause DM itself36. The primary neurological symptoms of hypercalcemia are weakness, disorientation, sleepiness and lethargy38. Additionally, the onset of seizures is thought to have been caused by vasoconstriction and hypertensive encephalopathy brought on by hypercalcemia55,56. Therefore, seizures, which may be the only presenting symptom, can be a fast-progressing neurologic sign of severe or acute electrolyte imbalances linked to diabetes mellitus. Additionally, hyperglycemia can cause damage to blood vessels in the brain, leading to the development of epilepsy. Individuals with epilepsy pose a higher risk of developing hyperglycemia due to antiepileptic therapy, which itself can cause insulin resistance and impair glucose metabolism57.
Supportive data: Several studies have investigated the relationship between epilepsy and hyperglycemia, particularly in individuals with type 1 diabetes mellitus (T1DM). These studies faced challenges in distinguishing unprovoked epileptic seizures from seizures caused by metabolic dysfunctions like hypoglycemia. In some instances, comprehensive clinical details or metabolic studies for all participants were not fully accessible. Despite these limitations, the research indicated that individuals with T1DM are at a higher risk of epilepsy compared to the general population, with the risk ranging from two to six times greater. This elevated risk persists even among patients below 18 years of age, although some research groups were unable to confirm this outcome.
The prevalence of epilepsy in individuals with T1DM varies between 8.7 and 21 per 1000 individuals, with T1DM occurring two to four times more frequently in patients with idiopathic generalized epilepsy compared to the general population. Additionally, two different cohorts, one in the United Kingdom and the other in Taiwan, found a higher incidence of epilepsy in patients with T1DM compared to control groups.
On average, T1DM typically develops 1.5-2.8 years prior to the onset of epilepsy. Specific risk factors for epilepsy occurrence in individuals with T1DM include a younger age and an earlier age of diabetes onset. The risk of ketoacidosis doubles in individuals with both T1DM and epilepsy, regardless of the antiepileptic drugs used for treatment. However, no notable differences were found in metabolic control, insulin treatment schedules and doses, or the presence of beta cell-specific autoantibodies between individuals with comorbid T1DM and epilepsy and those with T1DM alone.
Hyperglycemia poses a significant risk for focal seizures in individuals with T1DM due to heightened vulnerability to cerebrovascular dysfunctions. These dysfunctions are exacerbated by neuronal hyperosmolarity, dehydration and a subsequent decrease in cerebral blood flow. Certain cerebral structures, including the frontal or occipital cortex, amygdala and hippocampus, exhibit a lower seizure threshold in response to glucose level fluctuations, increasing the occurrence of seizures in T1DM patients, particularly those with pre-existing structural lesions disrupting regional neuronal activity.
The correlation between T1DM and an increased likelihood of ketoacidosis in epilepsy patients remains unclear and requires further investigation. This finding contradicts the perceived anticonvulsant effects attributed to ketosis, which are independent of diabetes-related factors such as those observed with ketogenic diets or specific antiepileptic treatments. Proposed mechanisms for this phenomenon include the use of carbonic anhydrase inhibitors in antiepileptic treatments or malfunctions in mitochondrial respiratory chain enzymes.
Hypoglycemia, whether acute or chronic, may serve as a triggering factor for seizures in T1DM patients undergoing insulin therapy. Individuals with recurrent hypoglycemic episodes have a heightened risk of epilepsy compared to euglycemic individuals. Acute seizures resulting from hypoglycemia are linked to functional and structural damage, causing increased cortical excitability due to the brain's insufficient energy supply from glucose. Preclinical studies suggest a potential role of glutamate-related excitotoxicity resulting from reduced glucose availability, leading to EEG abnormalities or unprovoked seizures. Adaptive mechanisms may be activated in cases of recurrent hypoglycemia, utilizing alternative energy sources such as lactate, which can increase susceptibility to epilepsy58.
Management: Regarding management, there are currently no specific criteria or guidelines for managing both hyperglycemia and epilepsy concurrently. However, recent literature suggests practical approaches, including regular blood sugar monitoring, lifestyle changes to improve glucose control, such as regular exercise, a healthy diet and stress-reducing activities. While antiepileptic drugs are commonly assumed necessary, recent studies emphasize the importance of antidiabetic therapy in managing hyperglycemia and epilepsy. Although antiepileptic drugs may be used, the focus remains on managing blood glucose levels and antiepileptic therapy should be adjusted as necessary to reduce the risk of hyperglycemia. For non-pharmacological therapy, recent research shows that the management of hyperglycemia in people with epilepsy should involve monitoring blood sugar levels regularly and making lifestyle changes to improve glucose control. This includes engaging in regular exercise, eating a healthy diet, avoiding triggers that can cause seizures and engaging in stress-reducing activities such as meditation and yoga28.
When it comes to pharmacologic management, recent studies showed that the role of antiepileptic drugs is not nearly as important as the role of antidiabetic therapy in patients with hyperglycemia and epilepsy. Most individuals with diabetes who have partial epilepsy are resistant to regularly prescribed antiepileptic medications despite the widespread belief that treating these patients with antiepileptic medicines is essential2,59. On the other hand, diazepam can be used to treat partial status epilepticus and certain DM patients with epilepsy have been shown to respond well to carbamazepine11. Anti-diabetic medications, however, are seen to be the most crucial elements in the management of the disease24. Even after antiepileptic medications are stopped, individuals can no longer have seizures when their blood glucose levels progressively return to normal. If antiepileptic drugs are used in treatment, studies still recommend a focus on managing blood glucose levels and antiepileptic therapy should be adjusted as necessary to reduce the risk of hyperglycemia21. The therapeutic approach for managing epilepsy in patients with type 1 diabetes (T1DM) is like that of patients without T1DM. The principles of diabetes therapy, including insulin use, nutritional recommendations and physical activity, can also have notable positive effects on seizures by ensuring optimal metabolic control59.
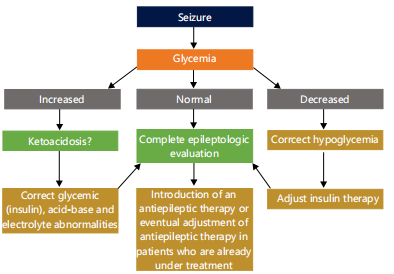
The ketogenic diet has been observed to effectively control seizures in a small number of documented patients with diabetes, without increasing the risk of hypoglycemia or complicated ketoacidosis28,60,61. A 3-year-old boy with myoclonic-astatic epilepsy, a 4-year-old boy with pyruvate dehydrogenase deficiency who developed type 1 diabetes mellitus (T1DM) after beginning the diet, a bilateral watershed infarcts in migrationdisorder were among the cases that were reported28,62. it might be difficult to discern between ketosis brought on by fasting and ketosis brought on by insulin shortage, the authors of these trials all stressed the significance of regularly monitoring insulin therapy and glucose levels27. The following illustration (Fig. 1) below summarizes potential pathways of treating and managing these patients.
|
CONCLUSION
There is compelling evidence for a tight association between hyperglycemia and epilepsy. Individuals with epilepsy are at a higher risk of developing hyperglycemia due to the use of antiepileptic medications. The management of these conditions involves monitoring blood sugar levels and electrolytes regularly, making lifestyle changes to improve glycemic control and adjusting both anti-diabetic and anti-epileptic medications to minimize the risk of both hyperglycemia and seizures. By working closely with healthcare providers, people with epilepsy and hyperglycemia can manage their conditions effectively and improve their quality of life.
SIGNIFICANCE STATEMENT
Significant diseases that are becoming recognized to be related to one another are hyperglycemia and epilepsy. The increased frequency of epilepsy and DM together is highlighted by epidemiological statistics, underscoring the necessity for customized therapeutic approaches. Thus, this research investigates the intricate relationship between hyperglycemia and epilepsy. Also, explores the pathophysiology of neurotransmitter activity changes, electrolyte imbalances and effects on neuronal excitability that are brought on by hyperglycemia. Clinical problems develop when DM coexists with epilepsy because some antiepileptic drugs might affect glycemic management and vice versa. The review focuses on the age of onset, risk factors and possible comorbidities as it investigates the epidemiology of these coexisting diseases.
REFERENCES
- Dhanasekaran, M., M. Almaghrabi, M. Majrashi, K. Abbott and J. Salamat et al., 2021. Antiepileptic effects of Oroxylum indicum extract in a valid (kainic acid-administered) rodent model of epilepsy. FASEB J., 35.
- Mastrangelo, M., V. Tromba, F. Silvestri and F. Costantino, 2019. Epilepsy in children with type 1 diabetes mellitus: Pathophysiological basis and clinical hallmarks. Eur. J. Paediatr. Neurol., 23: 240-247.
- Mani, V., M. Arfeen, H.A. Mohammed, H.A. Elsisi and S. Sajid et al., 2022. Sukkari dates seed improves type-2 diabetes mellitus-induced memory impairment by reducing blood glucose levels and enhancing brain cholinergic transmission: In vivo and molecular modeling studies. Saudi Pharm. J., 30: 750-763.
- Govindarajulu, M., P.D. Pinky, I. Steinke, J. Bloemer and S. Ramesh et al., 2020. Gut metabolite TMAO induces synaptic plasticity deficits by promoting endoplasmic reticulum stress. Front. Mol. Neurosci., 13.
- Deshpande, A.D., M. Harris-Hayes and M. Schootman, 2008. Epidemiology of diabetes and diabetes-related complications. Phys. Ther., 88: 1254-1264.
- Yang, E., K. Gavini, A. Bhakta, M. Dhanasekaran, I. Khan and K. Parameshwaran, 2018. Streptozotocin induced hyperglycemia stimulates molecular signaling that promotes cell cycle reentry in mouse hippocampus. Life Sci., 205: 131-135.
- Dhatariya, K.K., N.S. Glaser, E. Codner and G.E. Umpierrez, 2020. Diabetic ketoacidosis. Nat. Rev. Dis. Primers, 6.
- Rutters, F. and G. Nefs, 2022. Sleep and circadian rhythm disturbances in diabetes: A narrative review. Diabetes Metab. Syndr. Obesity, 15: 3627-3637.
- Nakanishi-Minami, T., K. Kishida, T. Funahashi and I. Shimomura, 2012. Sleep-wake cycle irregularities in type 2 diabetics. Diabetol. Metab. Syndr., 4.
- Parameswaran, G. and D.W. Ray, 2022. Sleep, circadian rhythms, and type 2 diabetes mellitus. Clin. Endocrinol., 96: 12-20.
- Marcovecchio, M.L., M.I. Petrosino and F. Chiarelli, 2015. Diabetes and epilepsy in children and adolescents. Curr. Diabetes Rep., 15.
- Skomro, R.P., S. Ludwig, E. Salamon and M.H. Kryger, 2001. Sleep complaints and restless legs syndrome in adult type 2 diabetics. Sleep Med., 2: 417-422.
- Resnick, H.E., S. Redline, E. Shahar, A. Gilpin and A. Newman et al., 2003. Diabetes and sleep disturbances: Findings from the sleep heart health study. Diabetes Care, 26: 702-709.
- Sridhar, G.R. and K. Madhu, 1994. Prevalence of sleep disturbances in diabetes mellitus. Diabetes Res. Clin. Pract., 23: 183-186.
- Buysse, D.J., C.F. Reynold, T.H. Monk, S.R. Berman and D.J. Kupfer, 1989. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res., 28: 193-213.
- Kalmbach, D.A., A.S. Cuamatzi-Castelan, C.V. Tonnu, K.M. Tran, J.R. Anderson, T. Roth and C.L. Drake, 2018. Hyperarousal and sleep reactivity in insomnia: Current insights. Nat. Sci. Sleep, 10: 193-201.
- Bonnet, M.H. and D.L. Arand, 1997. Hyperarousal and insomnia. Sleep Med. Rev., 1: 97-108.
- Riemann, D., K. Spiegelhalder, B. Feige, U. Voderholzer, M. Berger, M. Perlis and C. Nissen, 2010. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med. Rev., 14: 19-31.
- Bonnet, M.H. and D.L. Arand, 2010. Hyperarousal and insomnia: State of the science. Sleep Med. Rev., 14: 9-15.
- Duke, B.J., 2008. Pathogenic effects of central nervous system hyperarousal. Med. Hypotheses, 71: 212-217.
- Dafoulas, G.E., K.A. Toulis, D. Mccorry, B. Kumarendran and G.N. Thomas et al., 2017. Type 1 diabetes mellitus and risk of incident epilepsy: A population-based, open-cohort study. Diabetologia, 60: 258-261.
- Hossain, M.J., M. Al-Mamun and M.R. Islam, 2024. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci. Rep., 7.
- Liu, J., P. Zhang, Q. Zou, J. Liang and Y. Chen et al., 2023. Status of epilepsy in the tropics: An overlooked perspective. Epilepsia Open, 8: 32-45.
- Ramakrishnan, R. and R. Appleton, 2012. Study of prevalence of epilepsy in children with type 1 diabetes mellitus. Seizure, 21: 292-294.
- Chou, I.C., C.H. Wang, W.D. Lin, F.J. Tsai, C.C. Lin and C.H. Kao, 2016. Risk of epilepsy in type 1 diabetes mellitus: A population-based cohort study. Diabetologia, 59: 1196-1203.
- Åsvold, B.O., T. Sand, K.A. Hestad and M.R. Bjørgaas, 2011. Quantitative EEG in type 1 diabetic adults with childhood exposure to severe hypoglycaemia: A 16 year follow-up study. Diabetologia, 54: 2404-2408.
- Hauser, E., C. Strohmayer, R. Seidl, R. Birnbacher, A. Lischka and E. Schober, 1995. Quantitative EEG in young diabetics. J. Child Neurol., 10: 330-334.
- Keezer, M.R., J. Novy and J.W. Sander, 2015. Type 1 diabetes mellitus in people with pharmacoresistant epilepsy: Prevalence and clinical characteristics. Epilepsy Res., 115: 55-57.
- Fathallah, N., R. Slim, S. Larif, H. Hmouda and C.B. Salem, 2015. Drug-induced hyperglycaemia and diabetes. Drug Saf., 38: 1153-1168.
- Phabphal, K., K. Limapichat, P. Sathirapanya, S. Setthawatcharawanich and A. Geater, 2012. Characterization of glucose homeostasis and lipid profile in adult, seizure-free, epileptic patients in Asian population. Eur. J. Neurol., 19: 1228-1234.
- Sundari, S.K.K., M. Alturki, I. Steinke, J. Deruiter and S. Ramesh et al., 2022. Cardiovascular toxin-induced hyperglycemic and hypoarousal pathology-associated cognitive impairment: An in silico and in vivo validation. Cardiol. Plus, 7: 178-185.
- Gastaut, H., S. Lyagoubi, E. Mesdjian, J. Saier and S. Ouahchi, 1968. Generalized epileptic seizures, induced by “non-convulsant” substances. Epilepsia, 9: 311-316.
- Kirchner, A., J. Velíšková and L. Velíšek, 2006. Differential effects of low glucose concentrations on seizures and epileptiform activity in vivo and in vitro. Eur. J. Neurosci., 23: 1512-1522.
- Zhou, K., H. Yang, Z. Xie, W. Wang and Z. Qu, 2024. Genetic prediction of antihyperglycemic drug targets and risk of epilepsy: A mendelian randomisation study. BMC Pharmacol. Toxicol., 25.
- Sander, J.W., J. Novy and M.R. Keezer, 2016. The intriguing relationship between epilepsy and type 1 diabetes mellitus. Diabetologia, 59: 1569-1570.
- Mancardi, M.M., P. Striano, A. Giannattasio, M.G. Baglietto and L. Errichiello et al., 2010. Type 1 diabetes and epilepsy: More than a casual association? Epilepsia, 51: 320-321.
- Zhou, Y. and N.C. Danbolt, 2014. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm., 121: 799-817.
- O'Connell, M.A., A.S. Harvey, M.T. Mackay and F.J. Cameron, 2008. Does epilepsy occur more frequently in children with type 1 diabetes? J. Paediatr. Child Health, 44: 586-589.
- McCorry, D., A. Nicolson, D. Smith, A. Marson, R.G. Feltbower and D.W. Chadwick, 2006. An association between type 1 diabetes and idiopathic generalized epilepsy. Ann. Neurol., 59: 204-206.
- Singh, P., S.C. Rao and R. Parikh, 2014. Neonatal diabetes with intractable epilepsy: DEND syndrome. Indian J. Pediatr., 81: 1387-1388.
- McKeon, A. and J.A. Tracy, 2017. GAD65 neurological autoimmunity. Muscle Nerve, 56: 15-27.
- Liamis, G., H.J. Milionis and M. Elisaf, 2009. A review of drug-induced hypocalcemia. J. Bone Mineral Metab., 27: 635-642.
- Liamis, G., E.M. Rodenburg, A. Hofman, R. Zietse, B.H. Stricker and E.J. Hoorn, 2013. Electrolyte disorders in community subjects: Prevalence and risk factors. Am. J. Med., 126: 256-263.
- Liamis, G., H.J. Milionis and M. Elisaf, 2011. Hyponatremia in patients with infectious diseases. J. Infect., 63: 327-335.
- Palmer, B.F. and D.J. Clegg, 2015. Eelectrolyte and acid-base disturbances in patients with diabetes mellitus. N. Engl. J. Med., 373: 548-559.
- Liamis, G., E. Liberopoulos, F. Barkas and M. Elisaf, 2014. Diabetes mellitus and electrolyte disorders. World J. Clin. Cases, 2: 488-496.
- Moloney, T.C., I. Idris, P. Waters, S. Howell, A. Vincent, B. Lang and P. Maddison, 2016. Autoantibodies to glutamic acid decarboxylase in patients with epilepsy and their relationship with type 1 diabetes: A pilot study. J. Neurol. Neurosurg. Psychiatry, 87: 676-677.
- Rubega, M., G. Sparacino, A.S. Sejling, C.B. Juhl and C. Cobelli, 2016. Hypoglycemia-induced decrease of EEG coherence in patients with type 1 diabetes. Diabetes Technol. Ther., 18: 178-184.
- Mann, S.J., 2008. The silent epidemic of thiazide-induced hyponatremia. J. Clin. Hypertens., 10: 477-484.
- Spital, A., 1999. Diuretic-induced hyponatremia. Am. J. Nephrol., 19: 447-452.
- Darlow, B.A., 1977. Symptomatic hyponatraemia associated with tolbutamide therapy. Postgrad. Med. J., 53: 223-224.
- Kadowaki, T., R. Hagura, H. Kajinuma, N. Kuzuya and S. Yoshida, 1983. Chlorpropamide-induced hyponatremia: Incidence and risk factors. Diabetes Care, 6: 468-471.
- Pokhrel, B., S.W. Leslie and S.N. Levine, 2024. Primary Hyperparathyroidism. StatPearls Publishing, Treasure Island.
- Lemoine, S., L. Figueres, J. Bacchetta, S. Frey and L. Dubourg, 2022. Calcium homeostasis and hyperparathyroidism: Nephrologic and endocrinologic points of view. Ann. d'Endocrinologie, 83: 237-243.
- Chen, T.H., C.C. Huang, Y.Y. Chang, Y.F. Chen, W.H. Chen and S.L. Lai, 2004. Vasoconstriction as the etiology of hypercalcemia-induced seizures. Epilepsia, 45: 551-554.
- Smith, S.J.M., 2005. EEG in neurological conditions other than epilepsy: When does it help, what does it add? J. Neurol. Neurosurg. Psychiatry, 76: ii8-ii12.
- Schober, E., K.P. Otto, A. Dost, N. Jorch and R. Holl, 2012. Association of epilepsy and type 1 diabetes mellitus in children and adolescents: Is there an increased risk for diabetic ketoacidosis? J. Pediatr., 160: 662-666.e1.
- Tricò, D. and R.I. Herzog, 2017. Metabolic brain adaptations to recurrent hypoglycaemia may explain the link between type 1 diabetes mellitus and epilepsy and point towards future study and treatment options. Diabetologia, 60: 938-939.
- Ganelin-Cohen, E., D. Modan-Moses, R. Hemi, H. Kanety, B. Ben-Zeev and C.S. Hampe, 2016. Epilepsy and behavioral changes, type 1 diabetes mellitus and a high titer of glutamic acid decarboxylase antibodies. Pediatr. Diabetes, 17: 617-622.
- Luitse, M.J.A., G.J. Biessels, G.E.H.M. Rutten and L.J. Kappelle, 2012. Diabetes, hyperglycaemia and acute ischaemic stroke. Lancet Neurol., 11: 261-271.
- Yan, D., E. Zhao, H. Zhang, X. Luo and Y. Du, 2017. Association between type 1 diabetes mellitus and risk of epilepsy: A meta-analysis of observational studies. Drug Discoveries Ther., 11: 146-151.
- Hyllienmark, L., J. Maltez, A. Dandenell, J. Ludvigsson and T. Brismar, 2005. EEG abnormalities with and without relation to severe hypoglycaemia in adolescents with type 1 diabetes. Diabetologia, 48: 412-419.
How to Cite this paper?
APA-7 Style
Patel,
S., Kim,
S., Pathak,
S., Jeyabalan,
J.B., Liu,
K., Nadar,
R., Ren,
J., Moore,
T., Dhanasekarna,
M. (2024). Association Between Hyperglycemia and Epilepsy: From Clinical Implication to Therapeutics. Trends in Medical Research, 19(1), 199-207. https://doi.org/10.3923/tmr.2024.199.207
ACS Style
Patel,
S.; Kim,
S.; Pathak,
S.; Jeyabalan,
J.B.; Liu,
K.; Nadar,
R.; Ren,
J.; Moore,
T.; Dhanasekarna,
M. Association Between Hyperglycemia and Epilepsy: From Clinical Implication to Therapeutics. Trends Med. Res 2024, 19, 199-207. https://doi.org/10.3923/tmr.2024.199.207
AMA Style
Patel
S, Kim
S, Pathak
S, Jeyabalan
JB, Liu
K, Nadar
R, Ren
J, Moore
T, Dhanasekarna
M. Association Between Hyperglycemia and Epilepsy: From Clinical Implication to Therapeutics. Trends in Medical Research. 2024; 19(1): 199-207. https://doi.org/10.3923/tmr.2024.199.207
Chicago/Turabian Style
Patel, Shreya, Shannon Kim, Suhrud Pathak, Jeyaram Bharathi Jeyabalan, Keyi Liu, Rishi Nadar, Jun Ren, Timothy Moore, and Muralikrishnan Dhanasekarna.
2024. "Association Between Hyperglycemia and Epilepsy: From Clinical Implication to Therapeutics" Trends in Medical Research 19, no. 1: 199-207. https://doi.org/10.3923/tmr.2024.199.207

This work is licensed under a Creative Commons Attribution 4.0 International License.