Evaluation of the Bioactive Composition and in vitro Neuroprotective Potential of the Combined Curcuma longa and Rosmarinus Officinalis Ethanol Extract
| Received 22 Mar, 2024 |
Accepted 26 May, 2024 |
Published 27 May, 2024 |
Background and Objective: Oxidative stress and acetylcholinesterase activities have been implicated in the pathogenesis of neurological conditions leading to memory loss. The bioactive constituents derived from individual plants are sometimes insufficient to provide attractive substantial pharmacological action when compared to a combination of multiple herbs. This study was aimed at investigating the neuroprotective property of the combined ethanolic extract of Rosmarinus officinalis (rosemary) leaf and Curcuma longa (turmeric) rhizome by assessing the antioxidant activity (DPPH, FRAP and NO scavenging ability) and inhibitory activity of cholinergic enzyme (acetylcholinesterase inhibitory activity) of the combined extract in vitro. Materials and Methods: The polyherbal extract was prepared from the combined turmeric and rosemary leaf ethanol extract at a ratio of 3:1. The identification and characterization of bioactive composition of this combined extract was obtained using GC-MS (Gas Chromatography-Mass Spectrometry). Results: Sixty-five bioactive compounds were identified at various concentrations and retention times. The major compounds identified include; 1-Hexacosene (6.46%), Eicosane (4.06%), 6,11-Dimethyl-2,6,10-dodecatrien-1-ol (14.67%), 9-Octadecenoic acid (3.97%) and Trans-13-octadecenoic acid (4.46%). Results also showed that ethanol extract of combined rosemary leaf and turmeric rhizomes (polyherbal), rosemary extract singly, or turmeric extract singly exhibited DPPH radical scavenging abilities, nitric oxide scavenging abilities, reducing power (Fe3+-Fe2+) and lipid inhibitory activities in concentration-dependent manner. Current study findings indicated that the polyherbal contained a considerably higher DPPH radical scavenging ability, nitric oxide scavenging ability, reducing power (Fe3+-Fe2+) and lipid inhibitory activity than the single extracts. Furthermore, the polyherbal elicited a higher inhibitory effect on acetylcholinesterase (IC50 = 48.72 µg/mL) than standard drug physostigmine (49.9880 µg/mL). Conclusion: Hence, Curcuma longa rhizome in combination with Rosmarinus officinalis leaf was rich in biologically active components; thus, could be employed to formulate new plant-based pharmaceutical drugs for the management of neurological conditions like Alzheimer’s disease.
| Copyright © 2024 Nmeazi et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Alzheimer’s disease (AD), the primary contributor to dementia, is a degenerative neurological condition associated with aging that is increasingly posing significant challenges to public health and imposing substantial financial strain1,2. According to the Alzheimer's Association, it is predicted that AD will impact approximately 6.2 million individuals across all age groups in 2021. Among them, about 5.8 million people will be aged 65 and above and this number is expected to triple to 13.8 million by 2050 due to longer life expectancy and population growth3. Individuals with AD experience a decline in memory and cognitive functions and may undergo significant changes in their personalities. These transformations occur because of the gradual impairment and death of nerve cells responsible for storing and processing information. AD develops due to the malfunctioning of various biochemical pathways. Several factors associated with the disease’s progression, such as aggregated amyloid-β-peptide and tau protein, excessive transition metals, oxidative stress and reduced acetylcholine levels, have been linked to the pathology of AD3.
Oxidative stress has been implicated in the disease process in several neurodegenerative disorders, including Alzheimer’s disease4. The increased metabolic demand of the brain makes oxidative stress a significant contributor to chronic and acute brain diseases5-8. Researchers in the field of treating chronic and acute central nervous system (CNS) disorders and preventing secondary complications have increasingly focused on inhibiting the activity of NADPH-oxidase (NOX) as it plays a crucial role in oxidative stress in neurological disorders. Evidence has been accumulating to demonstrate that inhibiting NOX activity is neuroprotective and it has been shown that inhibiting NOX in circulating immune cells can enhance the neurological condition in various diseases. As a result, the inhibition of NOX has gained significant attention as a potential therapeutic approach9-11. While Aβ and tau are best known as the central proteins involved in AD pathogenesis, the link between the production of toxic Aβ peptides and oxidative stress is well documented. Studies have also demonstrated that oxidative stress plays a crucial part in tau hyperphosphorylation12. Also, studies have shown the essential role of oxidative stress in tau hyperphosphorylation.
Acetylcholinesterase is the main enzyme responsible for breaking down acetylcholine in the brain. Its role is to hydrolyze acetylcholine into two components, choline and acetate. This process effectively terminates the effects of acetylcholine at cholinergic synapses, which are involved in transmitting signals between neurons12. Hence, acetylcholinesterase serves as the focus for cholinesterase inhibitors employed to address the cholinergic decline in individuals with Alzheimer's disease. However, it’s important to note that these inhibitors are not capable of modifying the disease itself, nor can they simultaneously interfere with amyloid plaques, hinder tau hyperphosphorylation or offset oxidative stress by regulating mitochondrial bioenergetics and iron dysregulation13. It was reported in 2019 that several loss of lives were associated with the complications linked to AD. This has therefore led to cognitive efforts towards discovery of new drugs with multiple-target effects, aiming to tackle the diverse complications associated with AD14.
Several medicinal plants and their extracts have been investigated for their ability to counter the complications of AD15,16. Extensive research has been conducted on the utilization of Curcuma longa and its major yellow polyphenol compound, curcumin, found in the rhizomes of Curcuma longa, for the treatment of various diseases and conditions. This may be attributed to curcumin's ability to bind and interact with different cellular proteins. Curcumin has been studied for its potential to break beta sheets, reduce Aβ levels and exhibit antioxidant and anti-inflammatory properties. Furthermore, it has been discovered that curcumin can prevent the hyperphosphorylation of tau protein, which is responsible for the formation of neurofibrillary tangles17. The NADPH oxidase inhibitory potential of Curcuma longa has also been reported18,19.
However, despite the demonstrated effects of curcumin, its potential health benefits are constrained by challenges such as its limited solubility, poor absorption from the gastrointestinal tract, rapid metabolism and swift elimination from the body20,21. Considerable efforts have been dedicated to enhancing the absorption and bioavailability of curcuminoids present in this extract. Although a significant portion of ingested curcumin is eliminated unchanged through feces, the small fraction that is absorbed undergoes extensive conversion into water-soluble metabolites, namely glucuronides and sulfates. This extensive metabolism can impede their absorption into the body22,23.
One way to overcome this challenge is by employing combination treatments that involve other extracts to enhance bioavailability or inhibit additional pathways. Several strategies have been developed to improve the bioavailability of curcuminoids, building upon the traditional practice of consuming Curcuma longa rhizome in fat-based sauces, such as in a fat-rich yellow curry. These delivery strategies have shown significant potential in enhancing the bioavailability of curcuminoids24. The combined effects of Curcuma longa and Rosmarinus officinalis have been investigated in studies involving acute myeloid leukemia cells and breast cancer cells. These two compounds exhibited synergy in terms of their anti-proliferative effects and increased signaling for programmed cell death (apoptosis)25,26.
In a previous study conducted by Levine et al.27, it was demonstrated that Rosmarinus officinalis exhibited a synergistic effect when combined with Curcuma longa, resulting in a reduction in cellular proliferation. The study also revealed that treatment with Rosmarinus officinalis led to a significant increase in the cellular accumulation of curcumin. Specifically, there was approximately a 30% increase in curcumin accumulation in the C2 and D17 cell lines and a 4.8-fold increase in the CMT-12 cell line. This enhanced intracellular curcumin level potentially contributes to the observed synergy when using Curcuma longa and Rosmarinus officinalis in combination.
Carnosic acid, the active compound found in Rosmarinus officinalis, has the ability to impact multiple signaling pathways, some of which overlap with the pathways targeted by curcumin28. In a study conducted by Levine et al.29, it was noted that the safety profile of these commonly used feed ingredients, along with their consistent synergistic effects, make them potential candidates for inclusion in the diet as adjunctive treatments for dogs with neoplasia, provided that appropriate serum concentrations can be achieved. In a recent study by Mohamed et al.30, it was shown that the simultaneous administration of curcumin and rosemary essential oil resulted in an enhanced hepatoprotective effect. This effect was associated with an increase in the expression of both MEK1 and ERK1 proteins and elevated levels of curcumin in the plasma. These findings suggest a synergistic activity between these two natural products. Multiple studies have highlighted that curcumin faces challenges in crossing the blood-brain barrier and requires the use of bioavailability enhancers for optimal delivery31,32.
Merely having curcumin present in the bloodstream does not ensure its effective delivery to the brain, which is crucial for combating neurological disorders. The synergistic interactions between the compounds within individuals, mixtures, or medicinal plant extracts play a vital role in their therapeutic effectiveness33. Consequently, it is imperative to subject medicinal plant extract synergies to rigorous analysis methods and validate their efficacy through clinical trials. Furthermore, there is an ongoing need to better comprehend the specific bioactive compounds accountable for these effects and the fundamental mechanisms through which they interact, as our understanding in these areas remains incomplete34.
In this context, the present work investigates the bioactive constituents, antioxidant and acetylcholinesterase inhibitory potential of combined ethanol extract of Curcuma longa rhizome and Rosmarinus officinalis leaf as a treatment model for AD.
MATERIALS AND METHODS
Study location and duration: This Study was carried out from September, 2022 to February, 2023 at the Department of Biochemistry, Faculty of Science, Rivers State University.
Collection of plant materials: Plants materials including Curcuma longa rhizome and Rosmarinus officinalis leaf were obtained from a popular vegetable market at Obigbo, Oyibo LGA, Rivers State, Nigeria.
The identity of the plants was confirmed by a senior plant taxonomist at the Department of Plant Science and Biotechnology, Faculty of Science, Rivers State University. Voucher specimens were deposited in the herbarium of the Department of Plant Science and Biotechnology, Rivers State University.
Preparation of extracts: The collected plant’s leaves were dried under shade within one week on a laboratory bench and were thereafter pulverized to a coarse powder in a locally fabricated blender. For the preparation of extracts, the cold maceration technique used32 was adopted. Two hundred grams of the powdered sample was macerated in 1.5 liters of 96% ethanol within 48 hours and filtered, first with a clean handkerchief and then a filter paper. The filtrate so obtained was concentrated in a hot air oven at 40°C to obtain a strong congealed jelly-like residue for Curcuma longa which weighed 6.79 g and represented a 3.40% extract yield. For Rosmarinus officinalis, same procedures were followed but 9.35 g of a powder-like residue was obtained, representing an extract yield of 4.68%. The extracts so obtained were preserved in a refrigerator until use.
Percentage yields were calculated using the formula34:
Where:
| Q | = | Weight of extract obtained after extraction | |
| W | = | Weight of pulverized plant material |
The combination extract was prepared by mixing the Curcuma longa rhizome (turmeric ) and Rosmarinus officinalis leaf (rosemary) ratio of 1:1 such that for each 1 g of combined extract dissolved in 10 mL of distilled water, each prepared extract of Curcuma longa rhizome (turmeric ) and Rosmarinus officinalis (rosemary) contributed 0.50 g each.
Gas Chromatography-Mass Spectrometry (GC-MS) analysis of the extract extraction of phytochemicals: Briefly, 1 g of sample was weighed and transferred to a test tube and 15 mL of ethanol was added. The test tube underwent a reaction in a water bath at a temperature of 60°C for 60 min. Following the completion of the reaction, the resulting product was transferred from the test tube to a separatory funnel. The tube was effectively rinsed with 20 mL of ethanol, followed by 10 mL of cold water, 10 mL of hot water and 3 mL of hexane. All of these rinses were collected in the separatory funnel. The combined extract was subjected to three washes using 10 mL of a 10% v/v ethanol aqueous solution. The solution was then dried using anhydrous sodium sulfate, after which the solvent was evaporated. To enable analysis, the sample was dissolved in 1000 μL of ethyl acetate and 200 μL of this solution was subsequently transferred to a vial.
Quantification by gas chromatography: The phytochemical analysis was conducted using a BUCK M910 Gas chromatography instrument equipped with an HP-5MS column. The column dimensions were 30 m in length, 250 μm in diameter and 0.25 μm in film thickness. Spectroscopic detection was achieved through GC-MS with an electron ionization system utilizing high-energy electrons at 70 eV. Helium gas with a purity of 99.995% was employed as the carrier gas at a flow rate of 1 mL/min. The initial temperature of the system was set at 50-150°C, with a gradual increase of 3°C/min and a holding time of approximately 10 min. Finally, the temperature was raised to 300°C at a rate of 10°C/min. For injection, one microliter of the prepared 1% extract, diluted with the respective solvents, was introduced in a splitless mode. The relative quantities of chemical compounds present in each extract were expressed as percentages based on the peak areas observed in the chromatogram.
Identification of chemical constituents: The identification of bioactive compounds extracted from the samples was performed by comparing their GC retention times on an HP-5MS column and matching the spectra with data from computer software, specifically the standards from Replib and Mainlab data of GC-MS systems.
Antioxidant assay: The antioxidant activity of plant extracts was determined by different in vitro methods such as the DPPH free radical scavenging assay, ferric reducing power methods, nitric oxide scavenging capacity assay and lipid peroxidation inhibition assay. All the assays were carried out in triplicate and average values were considered.
2,2 Diphenyl-1-picryl-hydrazyl (DPPH) assay: The antioxidant activity of the extract was determined by the DPPH assay, as described earlier with some modifications33. Briefly, 200 μL of each extract (100-800 μg/mL) were mixed with 3.8 mL DPPH solution and incubated in the dark at room temperature for 1 hr. The absorbance of the mixture was then measured at 517 nm. Ascorbic acid and α-tocopherol were used as a positive control. The ability of the sample to scavenge DPPH radical was determined by:
Inhibitory concentration of 50% DPPH radical (IC50) was calculated as extracts' effective concentration scavenging half population of DPPH free radicals.
Nitric oxide scavenging capacity assay: The determination of the scavenging ability of nitric oxide involved the use of sodium nitroprusside, which is known to decompose in aqueous solution at physiological pH, resulting in the production of nitric oxide. Nitric oxide reacts with oxygen to generate nitrite ions, which can be quantified using Griess reagent. Scavengers of nitric oxide compete with oxygen, leading to a reduction in the generation of nitrite ions. In this study, a mixture of 10 mmol sodium nitroprusside in phosphate-buffered saline was combined with various concentrations (100, 200, 400 and 800 mg/mL) of the extracts and incubated for 150 min at room temperature. The assay control consisted of the same reaction mixture with distilled water instead of the extract. After the incubation period, 0.5 mL of Griess reagent, comprised of 1% sulfonamide, 2% H3PO4 and 0.1% N (1-naphthyl ethylene diamine dihydrochloride), was added and the absorbance was measured at a wavelength of 546 nm. Ascorbic acid (vitamin C) and α-tocopherol (vitamin E) were used as standards and the results were calculated accordingly33:
Where:
| Ac | = | Absorbance of control | |
| As | = | Absorbance of sample |
Inhibitory concentration (IC50) was also calculated as extracts’ effective concentration inhibiting half population of nitric oxide.
Ferric reducing power assay (FRAP): The determination of ferric reducing or antioxidant power was conducted following the previously described method34. In summary, 100 μL of the extract at concentrations ranging from 100 μg/mL to 800 μg/mL were combined with 2.5 mL of 200 mmol/L phosphate buffer (pH 6.6) and 2.5 mL of (1%) potassium ferricyanide. The mixture was then incubated at 50°C for 20 minutes. Subsequently, 2.5 mL of 10% trichloroacetic acid was added and the tubes were subjected to centrifugation at 10,000 rpm for 10 minutes. The upper layer (5 mL) was mixed with 5 mL of distilled water and 1 mL of 0.1% ferric chloride. The absorbance of the resulting reaction mixtures was measured at 700 nm. Ascorbic acid (vitamin C) and α-tocopherol (vitamin E) served as positive controls. The reducing ability was calculated as the percentage of inhibition.
Lipid peroxidation inhibition assay: The formation of a red chromogen di-adduct between TBA and MDA allows for spectrophotometric detection at 532 nm35. In this study, brain homogenate was prepared from normal rats weighing between 200-250 g. The brain was excised and washed with a 0.95 M NaCl solution. Homogenization of the brain tissue was carried out using a homogenizer at a temperature of -5°C with 5 mM phosphate-buffered saline (PBS) buffer at a ratio of 1:10 (homogenate to buffer) for 30 min. The resulting homogenate was then centrifuged for 15 min and the clear cell-free supernatant was collected for the investigation of in vitro lipid peroxidation.
In each test tube, 0.1 mL of the extract at different concentrations ranging from 100 to 800 μg/mL in dimethyl sulfoxide (DMSO) was added. To the test tubes, 1.4 mL of 50 mM PBS buffer and 0.5 mL of rat brain homogenate were also added. After incubation at 37°C for 15 min, the reaction was halted by adding 1 mL of 10% trichloroacetic acid (TCA) and 1 mL of 0.8% TBA. The mixture was then heated at 100°C for 15 min. Following cooling and centrifugation, the absorbance of the supernatants was measured at 532 nm. The results were expressed as μmol/mL MDA equivalent per gram of extract (μg/mL). The IC50 values were calculated based on the mean values obtained from three determinations. The ascorbic acid (vitamin C) and α-tocopherol (vitamin E) were used as standards at various concentrations ranging from 100 to 800 μg/mL.
Determination of acetylcholinesterase activity: Enzyme activity was measured by the method36. Enzyme activity reaction mixture (200 μL) consisted of 160 μL of 50 mM Tris HCl buffer, pH 7.4, with/without polyherbal extract followed by the addition of 10 μL enzyme (40-60 μg protein) from rat brain homogenate in 96-well plates. The contents were mixed and preincubated for 10 min at 25°C. Plates were pre-read at 412 nm using Synergy HT BioTek (USA) plate reader. The reaction was initiated by the addition of 10 μL of 1 mM DTNB and 3 mM substrate acetylthiocholine iodide. After 15 min incubation, absorbance was measured at 412 nm within 4-7 min. Control experiments were carried out for non-enzymatic hydrolysis by adding an enzyme after the addition of DTNB. Absorbance values were subtracted from the control and data was presented as percent inhibition of enzyme activity. Physostigmine was used as a standard inhibitor. All experiments were carried out in triplicate.
RESULTS
Chemical composition of mixed ethanol extract of Rosmarinus officinalis leaf and Curcuma longa rhizome: Table 1 shows the chemical composition of the mixed extract of Rosmarinus officinalis and the rhizome of Curcuma longa. Sixty-five chemicals were found in the mixed extract with five of them having a higher percentage composition, these chemicals include; 6,11-Dimethyl-2,6,10-dodicantrien-1-ol (14.67%), Eicosane (4.06%), 1-Hexacosene (6.46%), Trans-13-octandenoic acid (4.46%) and 9-octandecenoic acid (3.97%).
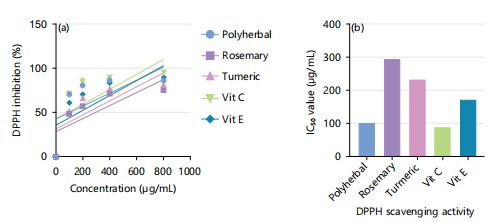
DPPH inhibition activity of combined ethanol extract of polyherbal, turmeric extract, rosemary, standard vitamin C and vitamin E: Figure 1a shows the free radical scavenging activity of polyherbal, turmeric extract and rosemary extract. Among the extracts, the polyherbal posed the highest activity. At a concentration of 200 μg/mL, the scavenging activity of polyherbal, rosemary and turmeric extract was 80.48±0.07, 56.94±0.07 and 66.30±0.30%, respectively whereas the activity of the standard’s ascorbic acid (vit C) and vit E at the same concentration was 81.04±0.44 and 70.15±0.30%, respectively.
|
| Table 1: | Chemical composition of mixed ethanol extract of Rosmarinus officinalis leaf and Curcuma longa rhizome | |||
| Peak | Retention time | Compound name | Composition (%) |
| 1 | 9.63 | Dodecane | 1.28 |
| 2 | 14.943 | Tetradecane | 0.38 |
| 3 | 17.41 | 2,4-di-tert-butylphenol | 1.23 |
| 4 | 19.257 | Dodecanoic | 3.11 |
| 5 | 19.566 | 9-Octadecene | 1.33 |
| 6 | 19.812 | Hexadecane | 1.46 |
| 7 | 23.48 | Tetradecanoic acid | 2.47 |
| 8 | 24.002 | 9-Eicosene | 0.43 |
| 9 | 24.213 | Sulfurous acid | 1.47 |
| 10 | 25.286 | Cyclohexane | 0.23 |
| 11 | 26.466 | Hexadecenoic acid | 0.22 |
| 12 | 27.497 | N-hexadecanoic acid | 3.3 |
| 13 | 28.205 | 1-Docosene | 1.74 |
| 14 | 28.205 | Heptacosane | 1.21 |
| 15 | 29.04 | Heptyl cyclohexane | 0.15 |
| 16 | 29.252 | 9-Octadecenoic acid | 0.34 |
| 17 | 29.575 | Methyl stearate | 0.13 |
| 18 | 29.75 | Cis-vaccenic acid | 5.95 |
| 19 | 29.868 | 9-Octadecenoic acid | 0.42 |
| 20 | 29.89 | Cis-vaccenic acid | 0.28 |
| 21 | 29.972 | Octadecanoic acid | 0.77 |
| 22 | 30.087 | Hexadecenoic acid | 0.62 |
| 23 | 30.242 | 1-Docosene | 1.07 |
| 24 | 30.308 | Carbonic acid | 0.2 |
| 25 | 30.394 | 5-Eicosene | 0.12 |
| 26 | 30.678 | 9-Octadecenoic acid | 0.12 |
| 27 | 30.787 | Cyclohexane | 0.17 |
| 28 | 31.482 | 1-Docosene | 0.99 |
| 29 | 31.525 | Carbonic acid | 0.21 |
| 30 | 31.623 | Carbonic acid | 0.13 |
| 31 | 31.746 | 12-Methyl-E, E-2,13-octadecadien-1-ol | 0.18 |
| 32 | 31.923 | Cyclohexane | 0.23 |
| 33 | 32.023 | 1-Decanol | 0.24 |
| 34 | 32.063 | Diisooctyl phthalate | 2.19 |
| 35 | 32.181 | Tricosane | 0.38 |
| 36 | 32.224 | Oleic acid | 0.29 |
| 37 | 32.257 | Oleic acid | 0.34 |
| 38 | 32.296 | Aspidospermidin-17-ol | 0.59 |
| 39 | 32.359 | Aspidospermidin-17-ol | 0.6 |
| 40 | 32.438 | 17-Pentatriacotene | 1.3 |
| 41 | 32.471 | 3-Eicosene | 0.62 |
| 42 | 32.506 | Oleic acid | 0.37 |
| 43 | 32.581 | Cyclotetracosane | 0.94 |
| 44 | 32.618 | 1-Nonadecane | 0.94 |
| 45 | 32.757 | Oleic acid | 3.82 |
| 46 | 32.789 | Cis-vaccenic acid | 1.97 |
| 47 | 32.883 | 9-Octadecenoic acid | 1.95 |
| 48 | 32.921 | Tetracosane | 2.2 |
| 49 | 32.971 | Oleic acid | 1.39 |
| 50 | 33.045 | 9-Octadecenoic acid | 3.97 |
| 51 | 33.09 | Cycloeicosane | 3.14 |
| 52 | 33.142 | Oleic acid | 2.27 |
| 53 | 33.191 | Trans-13-octadecenoic acid | 4.46 |
| 54 | 33.235 | 9-Octadecenoic acid | 3.33 |
| 55 | 33.297 | 9-Octadecenoic acid | 2.52 |
| 56 | 33.297 | Oleic acid | 2.21 |
| 57 | 33.333 | Oleic acid | 3.07 |
| 58 | 33.396 | 1-Hexacosene | 6.46 |
| 59 | 33.434 | Eicosane | 4.06 |
| 60 | 33.502 | 6,11-Dimethyl-2,6,10-dodecatrien-1-ol | 14.67 |
| 61 | 36.821 | Oleic acid | 0.01 |
| 62 | 36.821 | Octadecenoic acid | 0.02 |
| 63 | 37.022 | Cyclohexane | 0.03 |
| 64 | 37.081 | Oleic acid | 0.02 |
| 65 | 37.14 | Oleic acid | 0.01 |
The IC50 (extract concentration causing 50% scavenging values of the ethanolic extract of polyherbal, turmeric extract and rosemary extract were 101.074, 294.556 and 234.904 μg/mL, respectively (Fig. 1b). The IC50 values of the standards vit C and vit E were 89.59 and 173.269 μg/mL, respectively. The free radical scavenging activity of the extracts and standards was in the following order: Vit C>polyherbal extract>vit E>turmeric extract>rosemary extract.
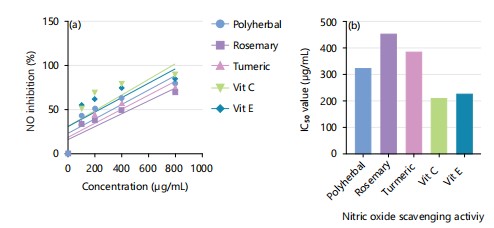
Nitric oxide scavenging activity of ethanolic extracts of polyherbal, turmeric extract, rosemary, standard vitamin C and vitamin E: Nitric oxide (NO) is a very unstable species and reacting with oxygen molecule produce stable nitrate and nitrite which can be estimated by using Griess reagent. In the presence of a scavenging test compound, the amount of nitrous acid will decrease which can be measured at 546 nm. The nitric oxide inhibitory activity of polyherbal, turmeric extract and rosemary extract was compared with vit C and vit E. At concentration of 200 μg/mL, the scavenging activity of polyherbal, rosemary extract and turmeric extract was 51.81±0.25, 38.63±0.22 and 45.21±0.49%, respectively whereas the nitric oxide inhibitory activity of vit C and vit E was 69.82±0.27 and 63.00±0.29%, respectively (Fig. 2a). The IC50 (extract concentration causing 50% inhibition) value of polyherbal, rosemary extract, turmeric extract, vit C and vit E were 323.372, 454.73, 387.41, 208.267 and 226.65 μg/mL (Fig. 2b). The polyherbal had a higher inhibitory activity than the single extracts and comparable inhibitory activity to that of the standards vit C and vit E. Figure 2b illustrates a decrease in the NO radical due to the scavenging ability of extracts such as percent inhibition of vit C>vit E>polyherbal>turmeric extract>rosemary extract.
Ferric reducing antioxidant power: Antioxidants play a crucial role in redox reactions by acting as reductants and neutralizing oxidants. In these reactions, one species undergoes reduction while another antioxidant undergoes oxidation. The antioxidant capacity of different Merremia tridentata extracts was evaluatedbased on their ability to reduce the TPTZ-Fe(III) complex to the TPTZ-Fe(II) complex.
|
| Table 2: | Percent ferric reducing antioxidant ability of ethanolic extracts of polyherbal, turmeric extract and rosemary | |||
| Concentration | Polyherbal | Rosemary | FRAP activity (%) Tumeric |
Vit C | Vit E |
| 100 (μg/mL) | 40.97±0.14 | 29.19±0.41 | 26.00±0.21 | 82.61±0.72 | 80.96±0.37 |
| 200 (μg/mL) | 49.39±0.19 | 31.53±0.70 | 35.58±0.21 | 93.27±0.30 | 89.87±0,16 |
| 400 (μg/mL) | 57.45±0.32 | 48.10±0.46 | 56.10±0.17 | 94.78±0.49 | 92.78±0.23 |
| 800 (μg/mL) | 64.99±0.40 | 56.22±0.53 | 61.03±0.52 | 96.07±0.64 | 98.11±0.61 |
| IC50 | 423.87 | 592.37 | 511.62 | 12.76 | 16.25 |
| Polyherbal: Combined ethanol extract of Rosmarinus officinalis leaf (rosemary) and Curcuma longa rhizome (turmeric). All experiments were performed in triplicate. Data are expressed as value represents the Mean±SEM (n = 3, p<0.05) for all tested dosages | |||||
| Table 3: | Lipid peroxidation activity of ethanolic extracts of polyherbal, turmeric extract and rosemary | |||
| Concentration | Polyherbal | Rosemary | MDA (u/mol) Tumeric |
Vit C | Vit E |
| 100 (μg/mL) | 4.62±0.02 | 4.10±0.01 | 4.54±0.02 | 3.28±0.10 | 4.13±0.02 |
| 200 (μg/mL) | 4.92±0.03 | 4.08±0.03 | 4.89±0.02 | 3.80±0.21 | 4.31±0.07 |
| 400 (μg/mL) | 6.15±0.03 | 4.29±0.03 | 7.92±0.04 | 4.11±0.42 | 4.83±0.10 |
| 800 (μg/mL) | 7.25±0.07 | 5.22±0.04 | 9.03±0.06 | 4.77±0.06 | 5.01±0.04 |
| IC50 | 6.73 | 10.97 | 6.71 | 10.93 | 10.99 |
| Polyherbal: Combined ethanol extract of Rosmarinus officinalis leaf (rosemary) and Curcuma longa rhizome (turmeric). All experiments were performed in triplicate. Data are expressed as value represents the Mean±SEM (n = 3, p<0.05) for all tested dosages | |||||
| Table 4: | Acetylcholinesterase inhibitory activity of ethanolic extracts of polyherbal and standard physostigmine | |||
| Concentration | Polyherbal inhibition (%) | Physostigm inhibition (%) |
| 0.2 (μg/mL) | 47.86±0.21 | 44.94±0.29 |
| 0.4 (μg/mL) | 52.85±0.67 | 47.93±0.21 |
| 0.6 (μg/mL) | 60.40±0.20 | 55.06±0.59 |
| 0.8 (μg/mL) | 66.60±0.35 | 64.46±0.12 |
| 1.0 (μg/mL) | 68.63±0.16 | 76.55±0.47 |
| IC50 | 48.72 | 49.99 |
| Polyherbal: Combined ethanol extract of Rosmarinus officinalis leaf (rosemary) and Curcuma longa rhizome (turmeric). All experiments were performed in triplicate. Data are expressed as value represents the Mean±SEM (n = 3, p<0.05) for all tested dosages | ||
The results are reported as the concentration of a substance that has the equivalent ferric-TPTZ to a percentage of Fe(II). The FRAP (Ferric Reducing Antioxidant Power) values for the polyherbal extract, rosemary extract and turmeric extract are presented in Table 2. Among the extracts, the polyherbal extract consistently exhibited a higher ferric reducing ability, as indicated by the IC50 value of 423.87 μg/mL, compared to the individual ethanol extracts of turmeric (511.62 μg/mL) and rosemary (592.37 μg/mL).
Lipid peroxidation inhibition assay: The lipid peroxidation inhibitory activity by the thiobarbituric acid assay (TBA assay) in vitro of polyherbal, turmeric extract, rosemary extract was compared with vit C and vit E. At concentration of 200 μg/mL, the inhibitory activity of polyherbal, turmeric extract and rosemary extract in the rat brain was 4.92±0.03, 4.89±0.02 and 4.08±0.03 MDA umol/mL, respectively whereas the lipid inhibitory activity of vit C and vit E was 3.80±0.21 and 4.31±0.07 MDA umol/mL, respectively. The IC50 (extract concentration causing 50% inhibition) value of polyherbal, vit C and vit E were 6.732, 10.925 and 10.998 μg/mL, respectively (Table 3). The polyherbal had a higher inhibitory activity than the single extract of rosemary and the standard vit C and vit E.
Acetyl cholinesterase inhibitory activity of ethanolic extracts of polyherbal and standard physostigmine: Table 4 shows the acetylcholinesterase inhibitory activity of polyherbal and the reference drug physostigmine in concentration-dependent manner (0.2–1.0 μg/mL). At a concentration of 0.4 μg/mL inhibitory activity of polyherbal was 52.85±0.67% whereas the activity of the reference drug physostigmine at the same concentration was 47.93±0.21% (Table 4). The IC50 value of the ethanolic extract of polyherbal and the reference drug physostigmine was 48.72 and 49.99 μg/mL, respectively (Table 4). The polyherbal had a higher acetylcholinesterase inhibitory activity compared with reference drug physostigmine.
DISCUSSION
The polyherbal extract was subjected to GC-MS chromatogram analysis, revealing the presence of sixty-five peaks, indicating the occurrence of thirty phytochemical constituents. By comparing the mass spectra of these constituents with the NIST library, five phytocompounds were successfully characterized and identified. Table 1 displays the different phytochemicals that contribute to the medicinal properties of the plants. The total antioxidant activities of plant extracts cannot be evaluated by using one single method, due to the complex composition of phytochemicals as well as of oxidative processes. Therefore, multiple methods should be employed to evaluate the total antioxidant activity37. In this present study the antioxidant activity of the ethanolic extracts of polyherbal, turmeric extract and rosemary were investigated by using DPPH scavenging assay ferric reducing power methods, nitric oxide scavenging capacity assay and lipid peroxidation inhibition assay. All methods have proven the effectiveness of the polyherbal extract compared to the reference standard antioxidants vit A and E.
The DPPH radical scavenging ability is one of the scientifically adopted standards in screening the antioxidant strength of samples. The ease of the reaction makes it widely utilized for evaluating free radical scavenging activity35. The free radical scavenging activity of various extracts was investigated by their capacity to reduce DPPH, which is a stable free radical. Any molecule capable of donating an electron or hydrogen to DPPH can react with it, leading to the decolorization of the DPPH absorption. The DPPH is a purple-colored dye that exhibits maximum absorption at 517 nm. Upon reaction with a hydrogen donor, the purple color of DPPH fades or disappears as it is converted to 2,2-diphenyl-1-picryl hydrazine, resulting in a decrease in absorbance. The DPPH test assesses the ability of the tested compound to serve as a scavenger of free radicals. The results of free radical scavenging activity of the different extracts along with the reference standards vit C and E are shown in Fig. 1.
The combined extract of rosemary and turmeric (polyherbal) exhibited a higher inhibition of DPPH activity than the single extract turmeric or rosemary and surpassed the activity of the standard commercial antioxidants, vit C and E dose-dependent manner. This suggested that the combined extract of rosemary and turmeric (polyherbal) contain compounds such as polyphenolics that can donate hydrogen easily.
Nitric oxide (NO) is a powerful and multifunctional inhibitor that affects various physiological processes, including smooth muscle relaxation, neuronal signaling, inhibition of platelet aggregation and regulation of cell-mediated toxicity. As a diffusible free radical, it serves as an influential molecule in diverse biological systems, acting as a neuronal messenger, promoting vasodilation and exhibiting antimicrobial and anti-tumor activities36. Production in excess of nitric oxide is associated with modifications (structurally and functionally) of cell components. Anti-radical agents are capable of quenching nitric oxide. Suppression of released nitric oxide may be partially attributed to direct nitric oxide scavenging, as the combined extracts of rosemary and turmeric (polyherbal) decreased the amount of nitrite generated from the decomposition of SNP than the single extract of turmeric or rosemary. The scavenging of nitric oxide by the extracts was increased in dose dependent manner.
The Ferric Reducing Antioxidant Power (FRAP) of the extracts was investigated. The reducing ability of a compound generally depends on the presence of reductants that have exhibited antioxidative potential by breaking the free radical chain and donating a hydrogen atom. The presence of reductants (i.e. antioxidants) in extracts of polyherbal, turmeric rhizome and rosemary leaves causes the reduction of the Fe3+/ferricyanide complex to the ferrous form. The reducing ability was presented against four different concentrations of each extract. The reducing power of the combined extract of rosemary leaf and turmeric rhizome (polyherbal) was more potent at all concentrations than the single extract of turmeric or rosemary compared to the standard vit C and vit E in a dose-dependent manner. The ethanol extract of turmeric rhizome showed the second highest reducing potency and the rosemary leaf extract showed the least efficacy towards reduction of Fe3+ to Fe2+.. Reducing properties is a defense system with two pathways obtainable to have an impact on this property are by electron transfer and hydrogen transfer37-39. The reducing capacity of the extracts could also be a sign of its potential antioxidant activities owing to the presence of reductants.
The inhibition of lipid peroxidation effect of the extracts was also investigated. Lipid peroxidation in cells results in the degradation of the lipid bilayer composing cell membranes37. Besides, lipid peroxidation end-products can further promote mutagenesis or protein oxidation, disturbing cellular homeostasis and their implications in human health have been extensively reviewed. Polyunsaturated fatty acids from rat brains react with oxygen to form malondialdehyde which reacts with thiobarbituric acid producing a pink coloration. A good antioxidant species should capably donate electrons to unstable free radicals inhibiting them from stealing free electrons from lipids in the cell membranes and consequently preventing oxidative degradation of lipids40,41. The extracts were tested against lipid peroxidation and concentrations capable of inhibiting lipid oxidation by 50% were calculated in μg/ mL. All the ethanolic extracts showed lipid peroxidation inhibition activity on brain rats with the polyherbal exhibiting a stronger inhibition against the formation of peroxides and hydroperoxides than the single extracts. The IC50 of the polyherbal (6.732 μg/mL) compared to the standard antioxidant vit C and E were 10.925 and 10.998 μg/mL, respectively. Flavonoids which are antioxidants have been reported to be effective inhibitors of lipid peroxidation41. Thus, the presence of more flavonoids in polyherbal may play an essential role in their antioxidant activity.
Current study findings on rosemary leaf and turmeric rhizome were in agreement with the reports42-44. They have reported varying levels of antioxidant and free radical scavenging properties of the extracts. However, tested the combined extract of rosemary leaf and turmeric rhizome showing more potent activity. The combined ethanolic extract of rosemary leaf and turmeric rhizome had strong antioxidant activity against all the free radicals investigated better than both single ethanolic extract of turmeric rhizome and leaf extract of rosemary.
Enzyme assays have become a valuable tool for assessing the potential health benefits of herbals, dietary supplements and nutraceuticals in the development and formulation of functional foods or phytopharmaceuticals45. Compounds that inhibit enzymes while also possessing antioxidant properties hold promise in the context of neurodegenerative diseases, as they can play a protective role against radical species45. Despite challenges related to absorption, metabolism and the precise mechanisms of action in vivo, screening for cholinesterase inhibitors derived from plants remains a valid approach for identifying compounds with significant biological activity45-47. Moreover, cholinesterase inhibitors remain the most effective available treatment for managing patients with Alzheimer's disease.
Therefore, to further confirm the neuroprotective potential of the polyherbal extract, acetylcholinesterase inhibitory activity of the polyherbal was investigated. Acetylcholinesterase, the predominant cholinesterase in the brain, hydrolyzes acetylcholine to choline and acetate, thereby terminating the effect of this neurotransmitter at cholinergic synapses48-50. The polyherbal acetyl cholinesterase inhibitory activity surpassed the activity of the standard commercial inhibitor physostigmine as shown in Table 3. The IC50 of the polyherbal was 48.7166 μg/mL compared to the physostigmine (49.9880 μg/ mL). This implies that the ethanol extract of the polyherbal offers a better pharmacological effect than the common synthetic drug. Acetyl cholinesterase inhibitory activity may be owing to the antioxidant properties of the polyherbal.
While individual plants have long been utilized in traditional medicine to treat various ailments, this study represents the first report on the combined antioxidant and acetylcholinesterase inhibitory activities of these plants. To date, there have been no scientific publications documenting the chemical constituents and nootropic effects of the combined extract. Consequently, this combination extract holds potential as a natural source of antioxidants, which could be of significant importance as therapeutic agents in Alzheimer's disease and other conditions associated with oxidative damage caused by free radicals.
CONCLUSION
Combined ethanol extract of rosemary leaf and turmeric rhizome (polyherbal) displayed remarkable inhibitory activity against selected cholinergic enzyme (acetylcholinesterase) and lipid peroxidation in rat brain while exhibiting antioxidant properties in vitro. This study has therefore shown that the combined extract of rosemary leaf and turmeric rhizome (polyherbal) contain multiple bioactive components with promising synergistic combinations to address the multiple dysregulated targets in Alzheimer disease and the challenge of bioavailability of turmeric extract.
SIGNIFICANCE STATEMENT
The need for general body well-being is paramount for effectiveness and good work output. The bioactive compounds derived from individual plants are sometimes inadequate for therapeutic purposes, hence the need for combined extract. Curcuma longa and Rosmarinus officinalis are two common plants with well-established therapeutic potencies, however, in combination, little or none is known in the past about its pharmacological potencies. This study found that the combination of Curcuma longa and Rosmarinus officinalis had bioactive compounds with high oxidative stress scavenging potencies, paramount for neuroprotection. The combined extract is also a rich source of antioxidants and, hence can be used both ameliorative and prophylactically.
REFERENCES
- Hachinski, V., K. Einhäupl, D. Ganten, S. Alladi and C. Brayne et al., 2019. Preventing dementia by preventing stroke: The Berlin Manifesto. Alzheimer's Dementia, 15: 961-984.
- Whiteford, H.A., A.J. Ferrari, L. Degenhardt, V. Feigin and T. Vos, 2015. The global burden of mental, neurological and substance use disorders: An analysis from the global burden of disease study 2010. PLoS ONE, 10.
- AA, 2016. 2016 Alzheimer's disease facts and figures. Alzheimer's Dementia, 12: 459-509.
- Vladimir-Knežević, S., B. Blažeković, M. Kindl, J. Vladić, A. Lower-Nedza and A. Brantner, 2014. Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the lamiaceae family. Molecules, 19: 767-782.
- Kim, G.H., J.E. Kim, S.J. Rhie and S. Yoon, 2015. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol., 24: 325-340.
- Gao, H.M., H. Zhou and J.S. Hong, 2012. NADPH oxidases: Novel therapeutic targets for neurodegenerative diseases. Trends Pharmacol. Sci., 33: 295-303.
- Ma, M.W., J. Wang, Q. Zhang, R. Wang, K.M. Dhandapani, R.K. Vadlamudi and D.W. Brann, 2017. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener., 12.
- Chen, H., Y.S. Song and P.H. Chan, 2009. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J. Cereb. Blood Flow Metab., 29: 1262-1272.
- Simonyi, A., P. Serfozo, T.M. Lehmidi, J. Cui and Z. Gu et al., 2012. The neuroprotective effects of apocynin. Front. Biosci., E4: 2183-2193.
- Barua, S., J.Y. Kim, M.A. Yenari and J.E. Lee, 2019. The role of NOX inhibitors in neurodegenerative diseases. IBRO Rep., 7: 59-69.
- Majdi, A., S. Sadigh-Eteghad, S.R. Aghsan, F. Farajdokht, S.M. Vatandoust, A. Namvaran and J. Mahmoudi, 2020. Amyloid-β, tau, and the cholinergic system in Alzheimer’s disease: Seeking direction in a tangle of clues. Rev. Neurosci., 31: 391-413.
- Ashraf, M., K. Ahmad, I. Ahmad, S. Ahmad, S. Arshad, S.M.A. Shah and F.U.H. Nasim, 2011. Acetylcholinesterase and NADH oxidase inhibitory activity of some medicinal plants. J. Med. Plants Res., 5: 2086-2089.
- Ehab, A., M. Ibrahim, M. Magdi, M. Ayman and N. Zidan et al., 2019. Alzheimer’s disease and its current treatments; Is there a possibility for a cure? Open J. Chem., 5: 013-019.
- Sharma, N.S., A. Karan, D. Lee, Z. Yan and J. Xie, 2021. Advances in modeling Alzheimer's disease in vitro. Adv. NanoBiomed Res., 1.
- Rubiano, A.M., N. Carney, R. Chesnut and J.C. Puyana, 2015. Global neurotrauma research challenges and opportunities. Nature, 527: S193-S197.
- Ozarowski, M., P.L. Mikolajczak, A. Bogacz, A. Gryszczynska and M. Kujawska et al., 2013. Rosmarinus officinalis L. leaf extract improves memory impairment and affects acetylcholinesterase and butyrylcholinesterase activities in rat brain. Fitoterapia, 91: 261-271.
- Onaolapo, A.Y., A.Y. Obelawo and O.J. Onaolapo, 2019. Brain ageing, cognition and diet: A review of the emerging roles of food-based nootropics in mitigating age-related memory decline. Curr. Aging Sci., 12: 2-14.
- Lee, S.H., S.A. Sancheti, M.R. Bafna, S.S. Sancheti and S.Y. Seo, 2011. Acetylcholineterase inhibitory and antioxidant properties of Rhododendron yedoense var. Poukhanense bark. J. Med. Plants Res., 5: 248-254.
- Basu, P., C. Maier and A. Basu, 2021. Effects of curcumin and its different formulations in preclinical and clinical studies of peripheral neuropathic and postoperative pain: A comprehensive review. Int. J. Mol. Sci., 22.
- Zhao, W.C., B. Zhang, M.J. Liao, W.X. Zhang, W.Y. He, H.B. Wang, and C.X. Yang, 2014. Curcumin ameliorated diabetic neuropathy partially by inhibition of NADPH oxidase mediating oxidative stress in the spinal cord. Neurosci. Lett., 560: 81-85.
- Okwakpam, F.N., A. Dokubo, M.O. Monanu and P.O. Uahomo, 2023. Cardioprotective effects of apocynin and curcumin against diclofenac-induced cardiotoxicity in male Wistar rats via inhibition of oxidative stress. Scholars Int. J. Biochem., 6: 86-98.
- Gupta, S.C., S. Patchva, W. Koh and B.B. Aggarwal, 2012. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol., 39: 283-299.
- Hewlings, S. and D. Kalman, 2017. Curcumin: A review of its' effects on human health. Foods, 6.
- Martins, C.A., G. Leyhausen, J. Volk and W. Geurtsen, 2015. Curcumin in combination with piperine suppresses osteoclastogenesis in vitro. J. Endodontics, 41: 1638-1645.
- Douglass, B.J. and D.L. Clouatre, 2015. Beyond yellow curry: Assessing commercial curcumin absorption technologies. J. Am. Coll. Nutr., 34: 347-358.
- Pesakhov, S., M. Khanin, G.P. Studzinski and M. Danilenko, 2010. Distinct combinatorial effects of the plant polyphenols curcumin, carnosic acid, and silibinin on proliferation and apoptosis in acute myeloid leukemia cells. Nutr. Cancer, 62: 811-824.
- Levine, C.B., J. Bayle, V. Biourge and J.J. Wakshlag, 2017. Cellular effects of a turmeric root and rosemary leaf extract on canine neoplastic cell lines. BMC Vet. Res., 13.
- Einbond, L.S., H.A. Wu, R. Kashiwazaki, K. He and M. Roller et al., 2012. Carnosic acid inhibits the growth of ER-negative human breast cancer cells and synergizes with curcumin. Fitoterapia, 83: 1160-1168.
- Levine, C.B., J. Bayle, V. Biourge and J.J. Wakshlag, 2016. Effects and synergy of feed ingredients on canine neoplastic cell proliferation. BMC Vet. Res., 12.
- Mohamed, M.E., N.S. Younis, H.S. El-Beltagi and O.M. Mohafez, 2022. The synergistic hepatoprotective activity of rosemary essential oil and curcumin: The role of the MEK/ERK pathway. Molecules, 27.
- Jäger, R., R.P. Lowery, A.V. Calvanese, J.M. Joy, M. Purpura and J.M. Wilson, 2014. Comparative absorption of curcumin formulations. Nutr. J., 13.
- Banji, D., O.J.F. Banji and K. Srinivas, 2021. Neuroprotective effect of turmeric extract in combination with its essential oil and enhanced brain bioavailability in an animal model. BioMed Res. Int., 2021.
- Banji, D., O.J.F. Banji, S. Dasaroju and K.C.H. Kranthi, 2013. Curcumin and piperine abrogate lipid and protein oxidation induced by D-galactose in rat brain. Brain Res., 1515: 1-11.
- Ulrich-Merzenich, G., D. Panek, H. Zeitler, H. Vetter and H. Wagner, 2010. Drug development from natural products: Exploiting synergistic effects. Indian J. Exp. Biol., 48: 208-219.
- Ijioma, S.N., E.E. Osim, A.A. Nwankwo, C.O. Nwosu and C.M. Ekeleme, 2019. Antioxidant potentials and effects on the hematology and osmotic fragility scores of a polyherbal formulation used in Southeast Nigeria. J. Basic Clin. Physiol. Pharmacol., 30.
- de Graaf, J.C., J.D. Banga, S. Moncada, R.M. Palmer, P.G. de Groot and J.J. Sixma, 1992. Nitric oxide functions as an inhibitor of platelet adhesion under flow conditions. Circulation, 85: 2284-2290.
- Ohkawa, H., N. Ohishi and K. Yagi, 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem., 95: 351-358.
- Ellman, G.L., K.D. Courtney, V. Andres Jr. and R.M. Featherstone, 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol., 7: 88-90.
- Zhao, H., W. Fan, J. Dong, J. Lu and J. Chen et al., 2008. Evaluation of antioxidant activities and total phenolic contents of typical malting barley varieties. Food Chem., 107: 296-304.
- Félix, R., P. Valentão, P.B. Andrade, C. Félix, S.C. Novais and M.F.L. Lemos, 2020. Evaluating the in vitro potential of natural extracts to protect lipids from oxidative damage. Antioxidants, 9.
- Didunyem, M.O., B.O. Adetuyi and I.A. Oyewale, 2020. Inhibition of lipid peroxidation and in-vitro antioxidant capacity of aqueous, acetone and methanol leaf extracts of green and red Acalypha wilkesiana Muell arg. Int. J. Biol. Med. Res., 13: 7089-7094.
- Kasote, D.M., S.S. Katyare, M.V. Hegde and H. Bae, 2015. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci., 11: 982-991.
- Pandey, M., K. Sonker, J. Kanoujia, M.K. Koshy and S.A. Saraf, 2009. Sida veronicaefolia as a source of natural antioxidant. Int. J. Pharm. Sci. Drug Res., 1: 180-182.
- Baptista, F.I., A.G. Henriques, A.M.S. Silva, J. Wiltfang and O.A.B. da Cruz e Silva, 2014. Flavonoids as therapeutic compounds targeting key proteins involved in Alzheimer’s disease. ACS Chem. Neurosci., 5: 83-92.
- Perrone, D., F. Ardito, G. Giannatempo, M. Dioguardi and G. Troiano et al., 2015. Biological and therapeutic activities and anticancer properties of curcumin. Exp. Ther. Med., 10: 1615-1623.
- Falade, A.O., G.I. Omolaiye, K.E. Adewole, O.M. Agunloye and A.A. Ishola et al., 2022. Aqueous extracts of bay leaf (Laurus nobilis) and rosemary (Rosmarinus officinalis) inhibit iron-induced lipid peroxidation and key-enzymes implicated in Alzheimer’s disease in rat brain-in vitro. Am. J. Biochem. Biotechnol., 18: 9-22.
- Ojo, O.A., A.B. Ojo, B.O. Ajiboye, O. Olaiya and A. Akawa et al., 2018. Inhibitory effect of Bryophyllum pinnatum (Lam.) Oken leaf extract and their fractions on α-amylase, α-glucosidase and cholinesterase enzyme. Pharmacogn. J., 10: 497-506.
- Choi, D.Y., Y.J. Lee, J.T. Hong and H.J. Lee, 2012. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer's disease. Brain Res. Bull., 87: 144-153.
- Sharma, N., M.A. Tan and S.S.A. An, 2021. Mechanistic aspects of apiaceae family spices in ameliorating Alzheimer’s disease. Antioxidants, 10.
- Sutalangka, C. and J. Wattanathorn, 2017. Neuroprotective and cognitive-enhancing effects of the combined extract of Cyperus rotundus and Zingiber officinale. BMC Complementary Altern., Med., 17.
How to Cite this paper?
APA-7 Style
Nmeazi,
O.F., Beverly,
O.M., Awolayeofori,
D., Nornubari,
N.J., Nkomadu,
V.D., Odinga,
T. (2024). Evaluation of the Bioactive Composition and in vitro Neuroprotective Potential of the Combined Curcuma longa and Rosmarinus Officinalis Ethanol Extract. Trends in Medical Research, 19(1), 136-150. https://doi.org/10.3923/tmr.2024.136.150
ACS Style
Nmeazi,
O.F.; Beverly,
O.M.; Awolayeofori,
D.; Nornubari,
N.J.; Nkomadu,
V.D.; Odinga,
T. Evaluation of the Bioactive Composition and in vitro Neuroprotective Potential of the Combined Curcuma longa and Rosmarinus Officinalis Ethanol Extract. Trends Med. Res 2024, 19, 136-150. https://doi.org/10.3923/tmr.2024.136.150
AMA Style
Nmeazi
OF, Beverly
OM, Awolayeofori
D, Nornubari
NJ, Nkomadu
VD, Odinga
T. Evaluation of the Bioactive Composition and in vitro Neuroprotective Potential of the Combined Curcuma longa and Rosmarinus Officinalis Ethanol Extract. Trends in Medical Research. 2024; 19(1): 136-150. https://doi.org/10.3923/tmr.2024.136.150
Chicago/Turabian Style
Nmeazi, Okwakpam,, Felicia, Otobo, Miebaka Beverly, Dokubo Awolayeofori, Nzor, Joyce Nornubari, Valentine Daddy Nkomadu, and Tamuno-Boma Odinga.
2024. "Evaluation of the Bioactive Composition and in vitro Neuroprotective Potential of the Combined Curcuma longa and Rosmarinus Officinalis Ethanol Extract" Trends in Medical Research 19, no. 1: 136-150. https://doi.org/10.3923/tmr.2024.136.150

This work is licensed under a Creative Commons Attribution 4.0 International License.