Salbutamol Oral Films: Design, Preparation and Evaluation Using Blends of Pectin and Mucilage of Dioscorea alata
| Received 09 Mar, 2024 |
Accepted 10 Jun, 2024 |
Published 11 Jun, 2024 |
Background and Objective: Salbutamol sulphate is indicated in the management of respiratory disorders such as asthma and bronchitis. However, how it is delivered can affect the onset of action, therapeutic effect obtained and patients’ adherence. This work prepared salbutamol sulphate oral dispersible films using blends of water yam mucilage (WYM) and pectin and compared its drug release profile to immediate release tablets of a commercial brand. Materials and Methods: The WYM was prepared from Dioscorea alatatubers and characterized but the pectin purchased. Preliminary compatibility test was done between the WYM, pectin and salbutamol sulphate using Fourier Transform infrared (FTIR) spectroscopy. Simple admixture blends of WYM with pectin in weight ratios (1:1, 1:4, and 2:3) were used as the film-forming polymer to produce oral films of salbutamol sulphate by the solvent evaporation method. The obtained films were evaluated for mechanical properties, disintegration time and drug release profile. The films were later re-evaluated after 10 months of storage in room conditions. The drug release profiles from the films were compared with oral tablets of marketed salbutamol sulphate. Results: The results showed that the moisture content and folding endurance of the oral films reduced after storage for 10 months, but the disintegration time increased. Both the oral films and the commercial tablet released all the drug content in 60 min. While the films followed zero-order release kinetics, the first-order kinetics best described the commercial tablet. After 10 months of storage, there was no significant difference in the release profile of the films except that F11 was now described by first-order kinetic. Conclusion: The WYM-pectin blends showed good potential as an oral film, providing an alternative delivery system for salbutamol, especially for patients with swallowing difficulty. The films possessed good mechanical properties, a stability profile and a drug release profile comparable to the oral tablets.
| Copyright © 2024 Effiong et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Salbutamol has been a drug of choice in symptomatic relief and prevention of respiratory disorders associated with airway constriction e.g., asthma, chronic obstructive pulmonary diseases, COPD and has been regarded as one of the safest essential medicines1. Generally presented as oral tablets, inhalations and injectibles, the use of salbutamol in these conventional dosage forms has its pharmaceutical limitations. Medicines delivered as oral films have increasingly become more popular for their convenience, trendy presentation and clinical advantage. Unlike tablets and capsules, oral films overcome swallowing reflex disorders common in pediatrics, geriatrics, or dysphagic patients and eliminating drug side effects as well as bypass the degradation by proteolytic enzymes in the gastrointestinal tract2-4. Also, unlike inhalations and injections which are dosage forms of choice, especially in the delivery of drugs (e.g., salbutamol) in emergencies, oral films require nonclinical expertise for administration and are safe even in chronic use5,6. Despite its several nomenclatures in literature, the oral film (soluble films, mucoadhesive films, wafers, oral strips, etc.,) are usually thin postage-stamp-sized oral solid dosage forms made from hydrophilic polymers. They are intended for rapid dissolution or dispersion in the mouth without the need for mastication, swallowing, or co-administration with water, to release the drug content for the desired therapeutic effect7,8. The avalanche blood supply of the or mucosa allows for the rapid absorption of drug content from the films into the systemic circulation, avoiding first-pass hepatic and gastrointestinal degradation5,9.
The choice, combination, or concentration of the polymer(s) in oral film formulation is critical considering its effects on the physicochemical and mechanical properties of the products10. Natural and synthetic polymers have been used alone or in combinations to achieve desired functionalities (e.g., drug loading capacity, mechanical strength, elasticity, reduced cost etc.) and outcomes in oral film formulations10-13. Cost-effectiveness, biocompatibility and less toxicity influence the choice of natural polymers over synthetic ones as carriers to develop films for drug delivery14.
Water yam mucilage is the mucus from the tubers of the Dioscorea alata plant. Although these tubers are consumed as food in West Africa, Asia and the Caribean, their mucilage is structurally composed of a complex carbohydrates-protein that is water soluble with highly branched structures of monosaccharide and uronic acids15,16. It has good rheological properties for stable emulsions at low concentrations and possesses good potential as a film-former15-17. Also a polymer from a plant, pectin is composed of residues of D-galacturonic acid that are partially or completely methyl esterified, likely responsible for its 3 and 4 values reported as a dissociation constant (pKa)18,19.
Used alone, pectin-based films for different applications have been reported to possess the limitation of weak mechanical strength, poor moisture and brittleness, but in combination with other polymers, these properties can be improved20. For example, pectin has been used with gellan gum to achieve improved swelling and mucoadhesive properties for buccal films; pectin blended with polyvinyl alcohol and incorporated with magnesium oxide (MgO) nanoparticles had improved thermal stability and mechanical strength; and films made from a combination of pectin and gelatin then irradiated with gamma rays have been reported to have improved mechanical properties18-21. A search in the databases by the authors reveals that there is yet to be oral film delivery system of the combination of water yam mucilage and pectin used for salbutamol. This study thus investigated the use of water yam mucilage blended with pectin at different weight ratios in the delivery of oral salbutamol films.
MATERIALS AND METHODS
Study area: This work was carried out in the laboratories of Pharmaceutics and Pharmaceutical Technology Department, the Pharmacognosy and Traditional Medicine Department and Pharmaceutical and Medicinal Chemistry Department, in the Faculty of Pharmacy, University of Uyo, Nigeria between January, 2022 and March 2023.
The materials used in this work included salbutamol powder (Hopkin and William Stan heat Essex, England), pectin (BDH chemicals Ltd, Poole, England), acetone (Fischer scientific international company, UK) and all other materials such as propylene glycol, aspartame, water yam mucilage, distilled water were of analytical grade.
Preparation of water yam mucilage: The WYM was obtained following the method reported by Onah and Shok22 with some modification. The yam tubers purchased from the market were peeled, washed with distilled water to remove debris and then grated. The resulting slurry was mixed with cold water (room temperature) in a weight ratio of 1:2, filtered using a muslin cloth and allowed to stand before decanting. To the decantate was added acetone at a volume ratio of 1:1 to precipitate the mucilage. The mixture was stirred and allowed to stand for some time to enable the mucilage to separate from the liquid. Thereafter, filtration was done to obtain the mucilage which was dried in the oven (Techmel Techmel, USA) for 24 hrs. Dried mucilage was size-reduced to form powders and kept for further use.
Evaluation of water yam mucilage: The mucilage was characterized in the solid state for densities, moisture content, particle size, flow properties (Hausner’s quotient, Carr’s index, angle of repose) and interaction with water (swelling index, viscosity, pH and solubility) following methods reported by Akpabio et al.23.
Photomicroscopy of the water yam mucilage: The particle size and morphology of the dry mucilage powder were determined using a digital MD500 armscope microscope eyepiece camera that was coupled to a light binocular microscope (Olympus CX21). Specifically, about 0.001 g of the dried mucilage powder was distributed onto a glass slide, with the addition of a drop of glycerine and safranin for colour contrast. A cover slide was placed on the prepared sample to fix sample onto the glass slide, then placed on the light microscope (Olympus CX21) stage to view. Snapshots of the views were taken at magnifications 10 and 40 and the diameter of at least 20 particles was determined.
Preparation of oral salbutamol films: Oral salbutamol films were prepared using the solvent-casting method24. Water yam mucilage and pectin blends were employed as film-forming polymers in the ratios by weight of 1:1, 1:4 and 2:3, respectively. Admixtures of the powders of the film-forming polymers were first prepared to obtain a uniform particulate blend and then a small quantity (7 mL) of warm laboratory obtained distilled water was added to form a polymer solution in one beaker. In another beaker, a solution of drug was prepared by dissolving salbutamol powder and aspartame in water (7 mL), followed by the addition of propylene glycol according to quantities in Table 1. The drug solution was then poured into the beaker with a polymer solution and made up to 20 mL with little agitation to mix but prevent introduction of air bubbles. After allowing the mixture to stand for 30 min for any entrapped air bubbles to escape, the solution mix was poured onto a clean Petri dish and placed in a laboratory oven (Techmel Techmel-5590) at 60oC to dry for a few hours. The films, on drying, were removed and cut with scissors to the desired sizes and shapes of 2 by 2 cm, that was equivalent to 4 mg24.
| Table 1: | Composition of each prepared batch of oral film for each Petri dish | |||
| Batches and quantities | |||
| Composition | F11 | F14 | F23 |
| Salbutamol (g) | 0.55 | 0.55 | 0.55 |
| WYM (g) | 0.5 | 0.2 | 0.4 |
| Pectin (g) | 0.5 | 0.8 | 0.6 |
| Propylene glycol (mL) | 0.3 | 0.3 | 0.3 |
| Aspartame (g) | 0.02 | 0.02 | 0.02 |
| Water (mL) | 20 | 20 | 20 |
| WYM: water yam mucilage | |||
Drug-polymer compatibility studies: A non-thermal method for compatibility test, Fourier Transform Infrared (FTIR) was used. A small mass (0.5 g) of the pure powdered drug covered with potassium bromide, KBr, disk (about drug to disk ratio of 1: 100) was scanned using FTIR spectrophotometer (Agilent Technologies FTIR machine model Cary 360, USA) in the frequency region of 4000-650 cm–1. The scans were also done on water yam mucilage, then polymer and drug-polymer (1:1) powdered mixtures. The generated absorption spectra were then analyzed for any chemical incompatibility between the drug and the polymers.
Statistical analysis: Statistical analysis was used to assess the effect of polymer composition and storage on the mechanical properties (disintegration, flexibility) of the films and their release kinetics using the One-way Analysis of Variance (ANOVA). The probability value, p<0.05 was considered significant at a 95% confidence interval.
Evaluation of the prepared films
Organoleptic examination: The physical appearance, colour, odour, texture and taste of the films were determined as described in literature25.
Weight variation: Ten films each from the three batches were randomly selected and weighed individually using an electronic weighing balance (Ohaus, Galaxy). The average weight for each film within each batch was determined26.
Film thickness: A micrometer screw gauge (KFW Scientific Industries Ambala Cantt India) was used to measure the thickness of the 5 randomly selected films. The measurement was taken at five points, the four edges and the centre of each film. The average thickness of selected films from each batch was determined2.
Folding endurance: Each of 5 randomly selected films was repeatedly folded at the same spot until a crack was observed. The crack was checked under bright light. The number of times the film was folded before any sign of crack was visible was taken as the folding endurance12. The mean value of folding endurance was determined.
pH: Each of 3 selected films for each batch prepared was placed in 2 mL of distilled water and allowed to stand for five minutes. The solution was made to be in contact with the electrodes of the pH meter (Jenway pH meter 3305) to determine the pH of the film and the average pH was calculated12.
Moisture content: A film was placed in the moisture content analyzer (LSC-60) and its initial weight was recorded. It was left in the analyzer until a constant weight was obtained. The percentage moisture content was calculated using the reported formula in Eq. 127:
| (1) |
The moisture content was determined for 3 samples of each batch and the average was noted.
Disintegration time: Exactly 3 randomly selected films were placed on a mesh wire in 20 mL of distilled water in a beaker and allowed to stand until it broke down to pass through the mesh. The time to achieve this was noted and the average time was determined28. This procedure was also carried out on the commercial tablets.
In vitro Dissolution study and release profile: The drug release profile of the oral salbutamol films was determined using USP basket-like dissolution apparatus (RCZ-6C3 Type Medicine Dissolving Instrument, China). A cut film size of 2 cm by 2 cm was placed in the basket and immersed in the 500 mL of 0.1N HCl dissolution medium maintained at 37±0.5oC as contained in the dissolution apparatus cylinder. In one of the cylinders, the commercial tablet was placed. Agitation speed was kept at 50 rpm. Exactly 10 mL aliquots were withdrawn at predetermined times of 5, 10, 20, 30, 40, 50 and 60 min and almost simultaneously replaced with equal volume of fresh medium to maintain a sink condition during the dissolution study. The absorbance of the withdrawn aliquot was read at a wavelength of 276 nm, the wavelength for peak absorbance for salbutamol, using an ultraviolet (UV) spectrophotometer (U2100 PC Shanghai, China). From the absorbance, the amount of drug released at each time was determined using the calibration curve of the pure drug.
The amount of drug release at the time intervals was subsequently subjected to four drug release kinetic models29; zero order (Q = kt) first order (lnQt = lnQ0-kt), Higuchi (Qt = kHt ½) and krsemeyer pppas ![]() to determine which model best describes the drug release and mechanism of release by comparing the value of the correlation coefficient. In the models Q, represent the amount of drug; k is the rate constant and t for the time referenced.
to determine which model best describes the drug release and mechanism of release by comparing the value of the correlation coefficient. In the models Q, represent the amount of drug; k is the rate constant and t for the time referenced.
Stability studies: The prepared oral films were stored at room temperature and wrapped in aluminium foil for 10 months after which it was evaluated for mechanical properties, water interaction and dissolution studies to ascertain their stability over time.
RESULTS
The properties of the water yam mucilage are presented in Table 2 whereas its morphology and microscopy are displayed in Fig. 1.
The salbutamol oral films prepared possessed similar organoleptic properties despite the different weight ratios of the film forming polymers as presented in Table 3 whereas the pH and mechanical properties of the films (recently prepared and after storage of 10 months) as well as that of the marketed tablets are shown in Table 4.
| Table 2: | Micromeritic and physicochemical properties of water yam mucilage | |||
| Properties | Results |
| pH | 6.68±0.08 |
| Solubility | Sparingly soluble in cold water |
| Soluble in warm water | |
| Viscosity (mPaS) | 5.60±0.84 |
| Bulk density (g/mL) | 0.58±0.01 |
| Tapped density (g/mL) | 0.73±0.03 |
| True density (g/mL) | 1.19±0.14 |
| Flow rate (g/s) | 2.06±0.34 |
| Hausner’s ratio | 1.26±0.04 |
| Carr’s index (%) | 20.47±2.69 |
| Angle of repose (°) | 45.48±3.20 |
| Moisture content (%) | 12.30±1.25 |
| Particle size (μm) | 4.92±4.54 |
| Table 3: | Organoleptic properties of the oral salbutamol films | |||
| Properties | F11 | F14 | F23 |
| Colour | Light pink | Light pink | Light pink |
| Odour | Odourless | Odourless | Odourless |
| Taste | Sweet | Sweet | Sweet |
| Appearance | Translucent | Translucent | Translucent |
| Surface texture | Smooth | Smooth | Smooth |

|
| Table 4: | Mechanical properties of the films | |||
| New oral films | After 10 months of storage | ||||||
| Properties | Marketed salbutamol tablet |
F11 | F14 | F23 | F11 | F14 | F23 |
| pH | - | 6.30±0.04 | 6.40±0.04 | 6.30±0.15 | 6.15±0.04 | 6.35±0.04 | 6.60±0.15 |
| Weight variation (mg) | 237±3.0 | 83±1.00 | 102±2.00 | 100±1.00 | 80±1.0 | 100±2.0 | 100±1.0 |
| Moisture content (%) | - | 8.40±0.31 | 7.10±0.96 | 9.91±0.66 | 6.60±0.31 | 7.51±0.96 | 6.90±0.66 |
| Folding endurance | - | 15.00±1.25 | 35.00±1.49 | 42.00±2.45 | 12.00±1.25 | 32.00 ±1.49 | 40.00±2.45 |
| Thickness (mm) | 3.17±0.05 | 0.22±0.01 | 0.25±0.01 | 0.24±0.01 | 0.22±0.01 | 0.25 ±0.01 | 0.24±0.01 |
| Disintegration time (s) | 1800±5 | 20.00±1.03 | 15.00±0.64 | 18.00±0.83 | 23.00 ±1.27 | 18.00 ±1.46 | 20.00±0.51 |
| Hardness (kg/cm2) | 4.00±0.10 | - | - | - | - | - | - |
| Friability (%) | 0.80±0.42 | - | - | - | - | - | - |
| Table 5: | Drug release kinetics and mechanisms of the salbutamol films | |||
| Newly prepared oral films | After 10 months of storage | |||
| Release mechanism | Release kinetics | Release mechanism | Release kinetics | |
| Formulation | exponent (n) | and R2 | and exponent (n) | and R2 |
| F11 | Non-fickian | Zero order | Non-Fickian diffusion | Zero and First |
| diffusion n = 0.66 | R2 = 0.976 | N = 0.52 | order R2 = 0.97 | |
| F14 | Non-fickian | Zero order | Non-Fickian diffusion | Zero order |
| diffusion n = 0.85 | R2= 0.981 | n = 0.58 | R2 = 0.979 | |
| F23 | Non-fickian | Zero order | Fickian diffusion | Zero order |
| diffusion N = 0.49 | R2 = 0.974 | n = 0.43 | R2 = 0.971 | |
| Commercial tablet | N =1.192 | First order | ||
| (standard) | R2 = 0.969 | |||
| F11: Mass ratio of mucilage to pectin1:1, F14: Ratio of mucilage to pectin 1:4 and F23: Ratio of mucilage to pectin 2:3 | ||||
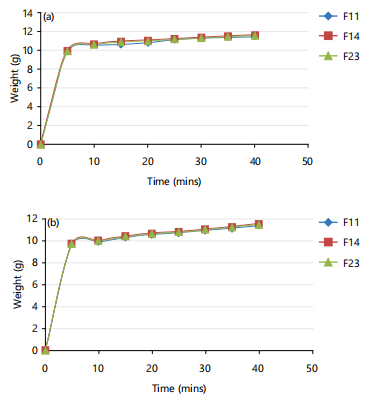
Figure 2(a-b) represent the results of the swelling index of the batches of prepared oral films in water after production and after storage for 10 months.
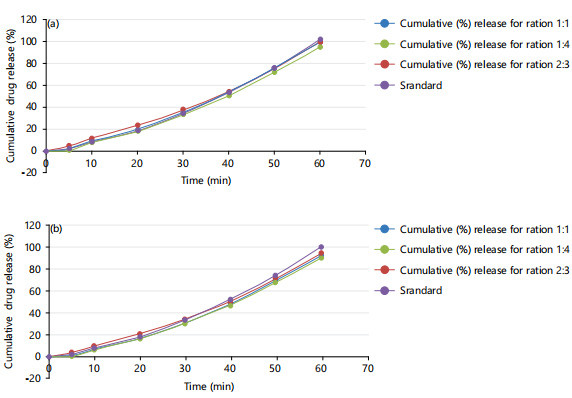
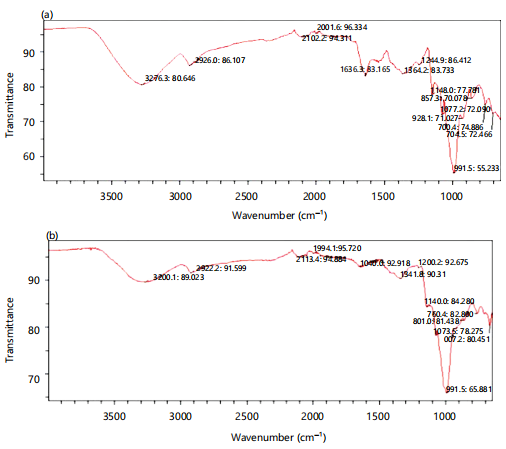
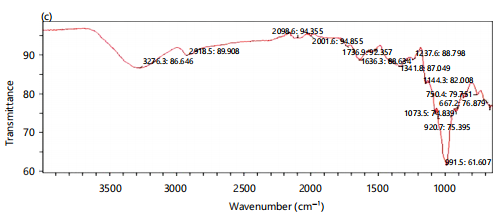
Figure 3(a-b) show the dissolution profile of the different batches of immediately prepared salbutamol oral films and those stored for 10 months. The dissolution studies were for 60 min. The drug-excipient compatibility study by the fourier transform infrared spectroscopy is resented in Fig. 4(a-c)
The release kinetics parameters and mechanism of drug release from the salbutamol oral films are presented in Table 5.
|
|
|
Water yam mucilage and pectin showing their characteristic stretchings and vibrations at specific wave numbers peculiar to the respective chemical compositions hence used to monitor for the salbutamol-excipient interaction and compatibility
DISCUSSION
The densities and micromeritics of dried water yam mucilage are presented in Table 2. Since bulk and tapped densities are properties of powders indicating their packing behaviour and flowability, they are influenced by the interparticulate interaction of the powders and the partticle shape but can affect operations involving the powder such as during manufacturing, bulk storage and compaction30,31. The density values of the mucilage and their derived properties, Hausner’s quotient and Carr’s index, reveal fair to passable flow. High values for Hausner's quotient and Carr’s index reflect cohesive powders and do not flow easily hence lower values are desirable. These flow indicators were well reflected in the directly measured flow rate which was determined to be 2.06 g/s. This value corroborates the conclusion that the mucilage powder possessed a passable flow, indicating the friction among the constituent particles.
Particle size and shape can influence powder flowability, compressibility and bulk density. When optimal, powder particle sizes allow the surface area interaction that encourages flowability. The particle size of the mucilage as determined was 4.92 μm likely contributing to the moderately high angle of repose, hence the fair to passable flow30. The mucilage with 12.3% moisture content, which is higher than that of enterolobium gum, will require good handling to prevent microbial contamination, chemical degradation and caution when considering use with moisture-sensitive drugs12.
The FTIR spectra to investigate possible undesirable interaction of salbutamol with the film-forming polymers showed a characteristic broad absorption band at 3265 cm–1 and a medium absorption band at 1640 cm–1, indicating O-H stretching and presence of N-H bending respectively (Fig 4b). Both functional groups are characteristic of pure salbutamol. The broad absorption band (3276cm–1) representing O-H stretching, though with a lower transmittance of 80% is equally present in Figure 4b, understandably so since this is a common functional group associated with mucilage. The two bands representing the functional groups of salbutamol were still present in the other spectra of drug-polymer mix (Fig 4c) revealing no observed shift or introduction of a new peak to the original spectra indicating no drug-polymer interaction. Salbutamol was compatible with the polymers.
Physical and mechanical properties of the films: Noteworthy to mention that the films made from the individual polymers were brittle and the mucilage, did not form a continuous film, but the blended in different weight ratios gave better films20. The obtained results for the weight of the films were in the range of 80-102 mg as shown in Table 4. Weight uniformity ensures the consistency of dosage units of formulations for therapeutic efficacy. The taste of the films was sweet, their colour was light pink, their texture was smooth and they were all translucent. The thickness of the films ranged from 0.22-0.25 mm and they had moderate folding endurance (flexibility) in the range of 15-42. The folding endurance increased in films with more concentration of pectin above 50% but appeared to deviate from such proportionality after an optimal concentration, hence is ranked F23>F14>F11.
The more dispersed the pectin in the mucilage in the film (especially in the range 50%
From the dissolution profile, within 60 min, the amount of drug released from the three formulations was above 90%, with the F14 showing the highest drug release of about 100%. No doubt, the different polymer ratios used for film production did not have a significant influence on the dissolution profile of the drug from the film. This in-vitro release profile of the prepared films is compared favorably with that of the oral tablet but the onset of action will be faster and bioavailability greater for oral film since its administration is through the buccal or sublingual path. The film formulations followed the zero-order release kinetics and the korsemeyer-peppas mechanism release models. Thus the films released a constant amount of drug content with time and not dependent on the amount of the drug remaining in the delivery system.
From the exponents of the korsemeyer-peppas equation (n-values range from 0.49 to 0.85), the non-Fickian release mechanism is indicated. Non-Fikian diffusion is commonly associated with porous media and swellable polymers34. The films had a very good swelling index (Fig 2 a-b), indicating their rapid fluid absorption capacity and expansion, giving room for a fast rate of drug release from the film by diffusion34. The commercial tablet however followed first-order kinetics.
Stability studies: After storage of prepared films in airtight wraps in a cupboard for 10 months, re-evaluation of the films revealed that the organoleptic properties and physical properties (e.g., weight, swelling index and thickness) were retained. However, an insignificant difference was observed in their disintegration time, moisture content and folding endurance of the films. In the dissolution study, it was observed that after 1 hr, the three formulations still had a cumulative percentage drug release of more than 90% and they retained the non-fickian release mechanism except for the F23 formulation whose mechanism of drug release now followed Fick’s diffusion, implying that the rate of drug diffusion from the film was variable.
CONCLUSION
In line with the expanding exploration into oral film technology as an advanced alternative drug delivery system for patients with swallowing disorders, salbutamol was successfully incorporated into oral film blends of water yam mucilage and pectin in this study. When used alone, both polymers gave poor oral films but their blends produced the film of good quality even when the drug was loaded on it. Water yam mucilage was found to exhibit film-forming ability only when used in combination with another polymer (pectin in this case). The pectin was optimal for improving mechanical properties of the mucilage at a weight ratio greater than 50% but less than 80%. The different weight ratios of polymers influenced the mechanical properties (folding endurance and disintegration time) of the films but not their dissolution profiles. Since the three film formulations compared favorably well with the conventional salbutamol tablets in terms of mechanical properties and release profile, salbutamol films from blends of water yam mucilage and pectin provide good prospects as an alternative means to administer the drug in the management of respiratory disorders and especially in patients having swallowing difficulties. This prospect must however also be examined in the light of in vivo studies.
SIGNIFICANCE STATEMENT
Oral dosage forms, inhalations and injectables have been employed in the management of respiratory conditions. The use of oral delivery can be a real challenge, especially in patients with dysphagia. The use of oral films, an advanced delivery system meets this challenge. However, there is concern about available film-forming polymers. This work employed a blend of native polymers to prepare a suitable film-forming polymer for salbutamol delivery as oral film. Formulated films compared favorably with commercial tablets in release profile even after storage for 10 months. Pectin and water yam mucilage showed good potential as a source of film-forming polymer showing good mechanical strength, stability and drug release.
ACKNOWLEDGMENT
The authors wish to express profound gratitude to Mr Emma Uduk and Mr Ifiok Ndem for their assistance in the Pharmaceutics and Pharmaceutical Technology Laboratory, Faculty of Pharmacy, University of Uyo, Nigeria.
REFERENCES
- Marques, L. and N. Vale, 2022. Salbutamol in the management of asthma: A review. Int. J. Mol. Sci., 23.
- Karki, S., H. Kim, S.J. Na, D. Shin, K. Jo and J. Lee, 2016. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci., 11: 559-574.
- Ekpa, E.D., U.R. Asuquo, A.A. Elijah, S. Ndiana-Abasi Ime and U.A. Ini, 2020. The oral film delivery- Application of nanotechnology and potential in medication adherence. GSC Biol. Pharm. Sci., 11: 34-51.
- Saxena, A. and T. Singh, 2022. Oral dissolving films: A comprehensive review on recent perspectives and current approach to effective drug delivery. J. Drug Delivery Ther., 12: 139-147.
- Bayrak, Z., C. Tas, U. Tasdemir, H. Erol, C.K. Ozkan, A. Savaser and Y. Ozkan, 2011. Formulation of zolmitriptan sublingual tablets prepared by direct compression with different polymers: In vitro and in vivo evaluation. Eur. J. Pharm. Biopharm., 78: 499-505.
- Aghera, N.J., S.D. Shah and K.R. Vadalia, 2012. Formulation and evaluation of sublingual tablets of losartan potassium. Asian Pac. J. Trop. Dis., 2: S130-S135.
- Tiwari, R.R., M.S. Umashankar, and N. Damodharan, 2018. Recent update on oral films: A bench to market potential. Int. J. Appl. Pharm., 10: 27-33.
- Mahboob, M.B.H., T. Riaz, M. Jamshaid, I. Bashir and S. Zulfiqar, 2016. Oral films: A comprehensive review. Int. Curr. Pharm. J., 5: 111-117.
- Özakar, R.S. and E. Özakar, 2021. Current overview of oral thin films. Turk. J. Pharm. Sci., 18: 111-121.
- Borges, A.F., C. Silva, J.F.J. Coelho and S. Simões, 2015. Oral films: Current status and future perspectives: I-Galenical development and quality attributes. J. Controlled Release, 206: 1-19.
- Kunte, S. and P. Tandale, 2010. Fast dissolving strips: A novel approach for the delivery of verapamil. J. Pharm. Bioallied Sci., 2: 325-328.
- Ayorinde, J.O., D.E. Effiong and M.A. Odeniyi, 2018. Design and evaluation of oral dissolving films of chlorpheramine from native and modified Enterolobium cyclocarpum gum. Afr. J. Biomed. Res., 21: 175-182.
- Shah, K.A., G. Li, L. Song, B. Gao and L. Huang et al., 2022. Rizatriptan-loaded oral fast dissolving films: Design and characterizations. Pharmaceutics, 14.
- Patil, S.B.S. and S. Daswadkar, 2021. A comprehensive review: Natural polymers used for fast dissolving mouth film. Int. J. Pharm. Sci. Rev. Res., 65: 14-21.
- Fortuna, D., S.S. Mardjan, T.C. Sunarti, E. Darmawati, S.M. Widayati and N. Purwanti, 2020. Extraction and characteristic of Dioscorea alata mucilage. IOP Conf. Ser.: Earth Environ. Sci., 542.
- Lozano, E.J., R.D. Andrade and J.G. Salcedo, 2018. Functional and rheological properties of yam (Dioscorea rotundata) mucilage. Adv. J. Food Sci. Technol., 15: 134-142.
- Huang, R., J. Xie, Y. Yu and M. Shen, 2020. Recent progress in the research of yam mucilage polysaccharides: Isolation, structure and bioactivities. Int. J. Biol. Macromol., 155: 1262-1269.
- Prezotti, F.G., I. Siedle, F.I. Boni, M. Chorilli, I. Müller and B.S.F. Cury, 2020. Mucoadhesive films based on gellan gum/pectin blends as potential platform for buccal drug delivery. Pharm. Dev. Technol., 25: 159-167.
- Sriamornsak, P., N. Wattanakorn and H. Takeuchi, 2010. Study on the mucoadhesion mechanism of pectin by atomic force microscopy and mucin-particle method. Carbohydr. Polym., 79: 54-59.
- Suhasini, M.R., K.M. Rajeshwari, S. Bindya, A.B. Hemavathi and P.M. Vishwanath et al., 2023. Pectin/PVA and pectin-MgO/PVA films: Preparation, characterization and biodegradation studies. Heliyon, 9.
- Jo, C., H. Kang, N.Y. Lee, J.H. Kwon and M.W. Byun, 2005. Pectin- and gelatin-based film: Effect of gamma irradiation on the mechanical properties and biodegradation. Radiat. Phys. Chem., 72: 745-750.
- Onah, P.O. and M. Shok, 2004. Extraction and characterization of mucilage from Crotalaria senegalensis Linn (Family-Fabaceae). Niger. J. Pharm. Res., 3: 106-109.
- Akpabio E.I., T.O. Uwah, D.E. Effiong and J. Godwin, 2020. Evaluating hydrocolloids of Sida acuta as sustained release matrix for ibuprofen tablet. Global J. Med. Res., 40: 16-21.
- Mashru, R.C., V.B. Sutariya, M.G. Sankalia and P.P. Parikh, 2005. Development and evaluation of fast-dissolving film of salbutamol sulphate. Drug Dev. Ind. Pharm., 31: 25-34.
- Uwah, T.O.O., E.I. Akpabio, T.C. Jackson, D.E. Effiong and E. Uduk, 2023. Potentials of Sterculia tragacantha Lindl seed husk gum as a release modifier in matrix tablet formulation. GSC Biol. Pharm. Sci., 25: 025-037.
- Karthik, D.R., H.S. Keerthy and R.P. Yadav, 2021. A review on fast dissolving oral films. Asian J. Pharm. Res. Dev., 9: 122-128.
- Chandramouli, M., R.P. Shivalingappa, V. Basavanna, S. Doddamani, D.C. Shanthakumar, S.R. Nagarajaiah and S. Ningaiah, 2023. Oral thin-films from design to delivery: A pharmaceutical viewpoint. Biointerface Res. Appl. Chem., 13.
- Kulkarni, P., D. Nirwan and R. Pacharane, 2020. Fast dissolving oral films a novel oral drug delivery system: An overview. Int. J. Sci. Res., 9: 1608-1611.
- Okunlola, A. and S.A. Adewusi, 2019. Development of theophylline microbeads using pregelatinizedbreadfruit starch (Artocarpus altilis) as a novel co-polymer for controlled release. Adv. Pharm. Bull., 9: 93-101.
- Amidon, G.E., P.J. Meyer and D.M. Mudie, 2017. Particle, Powder, and Compact Characterization. In: Developing Solid Oral Dosage Forms: Pharmaceutical Theory and Practice, Qiu, Y., Y. Chen, G.G.Z. Zhang, L. Yu and R.V. Mantri (Eds.), Academic Press, Cambridge, Massachusetts, ISBN: 9780128024478 pp: 271-293.
- Uwah, T.O., E.I. Akpabio, D.E. Ekpa, A.E. Akpabio and J. Godwin, 2018. Preliminary investigations into the physicochemical and compaction characteristics of modified starch of Discorea alata using diclofenac sodium tablet. Int. J. Pharm. Pharm. Sci., 10: 66-74.
- Bhyan, B., S. Jangra, M. Kaur and H. Singh, 2011. Orally fast dissolving film: Innovations in formulation and technology. Int. J. Pharm. Sci. Rev. Res., 9: 50-57.
- Heinemann, R.J.B., F.M. Vanin, R.A. de Carvalho, M.A. Trindade and C.S. Fávaro-Trindade, 2017. Characterization of low cost orally disintegrating film (ODF). Polímeros, 27: 48-54.
- Maheswari, K.M., P.K. Devineni, S. Deekonda, S. Shaik, N.P. Uppala and B.N. Nalluri, 2014. Development and evaluation of mouth dissolving films of amlodipine besylate for enhanced therapeutic efficacy. J. Pharm., 2014.
How to Cite this paper?
APA-7 Style
Effiong,
D.E., Akpabio,
E.I., Uwah,
T.O., Okon,
U., Kalu,
M.U. (2024). Salbutamol Oral Films: Design, Preparation and Evaluation Using Blends of Pectin and Mucilage of Dioscorea alata. Trends in Medical Research, 19(1), 208-219. https://doi.org/10.3923/tmr.2024.208.219
ACS Style
Effiong,
D.E.; Akpabio,
E.I.; Uwah,
T.O.; Okon,
U.; Kalu,
M.U. Salbutamol Oral Films: Design, Preparation and Evaluation Using Blends of Pectin and Mucilage of Dioscorea alata. Trends Med. Res 2024, 19, 208-219. https://doi.org/10.3923/tmr.2024.208.219
AMA Style
Effiong
DE, Akpabio
EI, Uwah
TO, Okon
U, Kalu
MU. Salbutamol Oral Films: Design, Preparation and Evaluation Using Blends of Pectin and Mucilage of Dioscorea alata. Trends in Medical Research. 2024; 19(1): 208-219. https://doi.org/10.3923/tmr.2024.208.219
Chicago/Turabian Style
Effiong, Daniel, Ekpa, Ekaete Ibanga Akpabio, Timma Oto-Obong Uwah, Uduak Okon, and Matthew Udochukwu Kalu.
2024. "Salbutamol Oral Films: Design, Preparation and Evaluation Using Blends of Pectin and Mucilage of Dioscorea alata" Trends in Medical Research 19, no. 1: 208-219. https://doi.org/10.3923/tmr.2024.208.219

This work is licensed under a Creative Commons Attribution 4.0 International License.